Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.457
Revised: June 8, 2005
Accepted: June 18, 2005
Published online: January 21, 2006
AIM: To investigate the expression of co-stimulatory molecule B7-H3 in gastric carcinoma and adenoma tissue as well as normal gastric tissue and to explore the relationship between B7-H3 expression and pathological features and prognosis of gastric carcinoma.
METHODS: B7-H3 expression was detected in 102 samples of human gastric carcinoma and 10 samples of gastric adenoma and 10 samples of normal gastric tissue by immunohistochemical assay. Correlation between the expression of B7-H3 and the patients’age, sex, gastric carcinoma locus, tumor size, tissue type, tumor infiltration depth, differentiation degree, lymph node metastasis, and survival time was analyzed.
RESULTS: B7-H3 was expressed in all gastric adenoma samples and in 58.8% samples of gastric carcinoma. B7-H3 expression in gastric carcinoma samples was not related with the patients’ age, sex, lymph node metastasis, and tumor size (P > 0.05), but with the survival time, infiltration depth of tumor and tissue type.
CONCLUSION: Detection of B7-H3 expression in gastric carcinoma tissue is beneficial to the judgment of the prognosis of gastric carcinoma patients and the choice of treatment.
- Citation: Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 2006; 12(3): 457-459
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.457
Tumor immune response is a very complicated physiological process involving many immune cells and molecules including membranous molecules and dissolubility factors. Studies indicate that a group of cell membrane molecules (co-stimulatory molecules) play a very important role in tumor immune response in the place of adjusted expression, interaction, and signal transmission. Co-stimulatory molecules are divided into two groups: TNF-TNF receptor superfamily and immunoglobulin superfamily. B7 family can transmit signals to co-stimulatory molecules of T cells[1]. B7RP-1 (B7H, B7-H2), B7-H1 (PD-L1), B7-DC (PD-L2) and B7-H3 have been found and make it more complex to adjust the effects of this family[2,3]. B7-H3 is a new co-stimulatory member of the B7 family and shares 20-27% identical amino acids with other members of the B7 family[4]. It was reported that B7-H3 could stimulate CD4+ and CD8+T cells to increase the activity of CTL[5]. B7-H3 is regarded as a positive regulation molecule. To our knowledge, expression of B7-H3 in gastric carcinoma has not been previously demonstrated.
From 1998 to 1999, 102 samples were collected from gastric cancer patients who had undergone surgery in our hospital. The tumor was located in the mucosa in 10 cases, in shallow muscularis in 20 cases and in deep muscularis in 72 cases. The tumors were classified into well-, moderately- and poorly-differentiated adenocarcinomas. None of the patients received chemotherapy or radiotherapy before the surgery. We also investigated 10 specimens from gastric adenoma patients who had undergone surgery between 1998 and 1999.
The study was approved by the ethics committee of our hospital and all patients gave their written informed consent prior to enrolment.
Labeling was carried out with Elivision™ plus kit. The samples were fixed in formalin, embedded with paraffin wax and cut into 3-µm-thick sections. The sections were dewaxed and rinsed and washed thrice with PBS (pH 7.4) for 3 min. The antigen of tissue was repaired and one drop or 50 µL of 3% hydrogen peroxidase solution was added to each section and incubated at room temperature for 10 min to eliminate endogenous peroxidase activity. The sections were washed thrice with PBS for 3 min again. PBS was discarded; one drop or 50 µL of primary antibody was added to each section and incubated at room temperature for 10 min or placed overnight at 40 °C. The sections were washed thrice with PBS for 5 min. PBS was discarded, one drop or 50 µL of polymer accentuator (reagent A) was added to each section and incubated at room temperature for 20 min. After being washed thrice with PBS for 3 min each time, PBS was discarded and one drop or 50 µL of enzyme-labeled anti-mouse/rabbit polymer (reagent B) was added to each section and incubated at room temperature for 30 min. The sections were washed thrice with PBS for 3 min each time. PBS was discarded; two drops or 100 µL of freshly prepared DBA coloration fluid was added to each section and examined under microscope for 3-10 min. All the samples were fixed in formalin, embedded with paraffin wax and cut into 3-µm-thick sections. The samples were assessed blindly by calculating the average ratio of positive cells in 10 vision fields (the plasma was stained brown-yellow) under a 400× microscope. If the average positive cell ratio was more than 20%, the sample was considered positive.
Difference between the groups was evaluated by χ2 test using SPSS version 10.0 for Windows and the relationship between the prognosis and various factors was evaluated by multivariable logistic regression. All P values were based on two-sided testing. P < 0.05 was considered statistically significant.
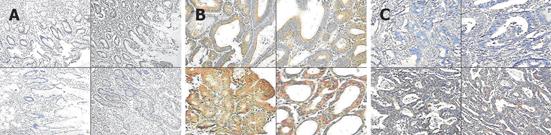
B7-H3 was positively expressed in cell membrane and cytoplasm of gastric cancer and adenoma cells (Figure 1). B7-H3 was expressed in all the 10 specimens of gastric adenoma tissue and in 58.8% samples of gastric cancer tissue.
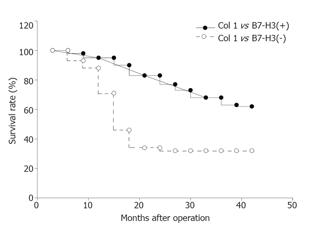
B7-H3 expression in gastric carcinoma tissue was not related with the patients’age and sex (P > 0.05, Table 1). The association of B7-H3 expression with the survival time after surgery indicated that B7-H3 expression was related to the prognosis of patients (Figure 2). The positive rate (74.5%) of B7-H3 expression was higher in gastric cancer patients who survived more than 5 years than in those who survived less than 2 years (43.1%, P < 0.01).
| Features | Total cases (n) | Positive cases (n) | Positive rate (%) |
| Tumor location | |||
| Top 1/3 layer of stomach | 31 | 19 | 61.2 |
| Middle 1/3 layer of stomach | 30 | 17 | 56.7 |
| Bottom 1/3 layer of stomach | 41 | 24 | 58.5 |
| Degree of differentiation | |||
| Well-differentiated | 72 | 48 | 66.7 |
| Poorly-differentiated | 30 | 12 | 40.0 |
| Infiltration depth without | |||
| Infiltration at deep muscular layer | 30 | 23 | 76.7 |
| With infiltration at deep muscular layer | 72 | 37 | 51.3 |
| Lymph node metastasis | |||
| Negative | 54 | 33 | 61.1 |
| Positive | 48 | 27 | 56.3 |
| Survival time | |||
| <2 years | 51 | 22 | 43.1 |
| >5 years | 51 | 38 | 74.5 |
| Primary tumor size | |||
| <5 cm | 55 | 35 | 63.6 |
| >5 cm | 47 | 25 | 53.2 |
B7-H3 expression was not related with lymph node metastasis, tumor location and size (P > 0.05), but with survival time, infiltration depth of tumor and histology type (Table 1).
Univariate analysis suggested that the infiltration depth of tumor, survival time of patients and histology type of gastric cancer were related to B7-H3 expression. After adjustment for these factors, gastric cancer patients with high intratumor B7-H3 expression could survive 2-fold longer than those with low intratumor B7-H3 expression (risk ratio = 2.803; 95%CI = 1.051-7.477; P = 0.040), suggesting that B7-H3 expression might be an independent factor affecting the survival time of gastric cancer patients.
Human B7-H3 is also known as B7 relative protein 2 (B7RP-2) and its gene map on chromosome 15 is composed of seven exons and six introns. Mature B7-H3 protein has 316 amino acids and its molecular weight is 45-66 ku. It belongs to immunoglobulin superfamily and is a type I transmembrane protein composed of extracellular, transmembrane and intracellular regions. B7-H3 has been recently identified as a new co-stimulatory member of the B7 family and shares 20-27% identical amino acids with other members of the B7 family[4]. It is extensively expressed in non-lymphoid tissues including the heart, liver, prostate, placenta, testis, pancreas, small, and large intestine and also in some tumor cell lines such as G361, HeLa S3, K562, A546, and SW480. B7-H3 was expressed in all the 10 specimens of gastric adenoma tissue and in 58.8% samples of gastric cancer tissue. We also found that B7-H3 was highly expressed in some epithelial tumor cell lines such as M435, A549, and H01299. A recent study found that B7-H3 can stimulate CD4+ and CD8+T cells to increase the activity of CTLs[5]. In addition, B7-H3 increases the secretion of IFN-γ and can upregulate IL-8 and TNF-α. When B7-H3Ig is blocked by anti-B7-H3 multi-clone antibody, secretion of IFN-γ by DCs can be downregulated, indicating that B7-H3 is a positive regulatory molecule.
Our study suggested that B7-H3 expression in gastric carcinoma tissue was not related with the age and sex of patients, lymph node metastasis and size of tumor but with the survival time of patients, infiltration depth and histology type of tumor. The positive rate (74.5%) of B7-H3 expression was higher in gastric cancer patients who survived more than 5 years than in those who survived less than 2 years (43.1%), suggesting that B7-H3 expression is an independent factor affecting the survival time of gastric cancer patients and that B7-H3 might act as a positive regulatory factor in tumor immunology.
Sun et al.[6] showed that intratumor injection of a mouse B7-H3 pcDNA3 expression plasmid leads to complete regression of 50% tumors and can significantly inhibit tumor growth. Mice with their tumors completely regressed can resist a challenge with parental tumor cells. B7-H3-mediated anti-tumor immunity is mediated by CD8(+) T and NK cells, rather than CD4(+) T cells. These results indicate that B7-H3 interactions play a role in regulating cell-mediated immune responses against cancer.
However, Suh et al.[7] found that B7-H3 inhibits immune response by inhibiting type 1 [T(H)1] responses and production of IFN-γ. A recent study showed that B7-H3 induces T cell proliferation and IFN-γ production through non co-stimulatory pathways[8], indicating that B7-H3 may have more than one receptor on activated T cells.
Because the known receptor of B7-H3 has not been found, its function in the immune response is not clear. B7-H3 is extensively expressed in peripheral tissues, suggesting that it may play an important role in inflammation and transplantation immune response. B7-H3 is highly expressed in epithelial tumor cell lines and positively related to the prognosis of gastric cancer patients, indicating that B7-H3-deficient expression in tumor tissues may be closely associated with tumor immune escape.
In conclusion, B7-H3 is related to the development of gastric cancer and acts as an independent index for the diagnosis and prognosis of gastric cancer. Further study is needed to explore the exact relationship between B7-H3 and gastric cancer.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1965] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 3. | Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 697] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Ayrapetov MK, Lee S, Sun G. Expression, purification, and biochemical characterization of Chk, a soluble protein tyrosine kinase. Protein Expr Purif. 2003;29:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |