Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.388
Revised: June 8, 2005
Accepted: June 24, 2005
Published online: January 21, 2006
AIM: To investigate the role of nuclear factor kappa B (NF-κB) in the pathogenesis of lung injury induced by intestinal ischemia/reperfusion (I/R), and its effect on intercellular adhesion molecule-1 (ICAM-1) expression and neutrophil infiltration.
METHODS: Twenty-four Wistar rats were divided randomly into control, I/R and pyrrolidine dithiocarbamate (PDTC) treatment groups, n = 8 in each. I/R group and PDTC treatment group received superior mysenteric artery (SMA) occluding for 1 h and reperfusion for 2 h. PDTC group was administrated with intraperitoneal injection of 2% 100 mg/kg PDTC 1 h before surgery. Lung histology and bronchia alveolus lung fluid (BALF) protein were assayed. Serum IL-6, lung malondialdehyde (MDA) and myeloperoxidase (MPO) as well as the expression level of NF-κB and ICAM-1 were measured.
RESULTS: Lung injury induced by intestinal I/R, was characterized by edema, hemorrhage and neutrophil infiltration as well as by the significant rising of BALF protein. Compared to control group, the levels of serum IL-6 and lung MDA and MPO increased significantly in I/R group (P = 0.001). Strong positive expression of NF-κB p65 and ICAM-1 was observed. After the administration of PDTC, the level of serum IL-6, lung MDA and MPO as well as NF-κB and ICAM-1 decreased significantly (P < 0.05) when compared to I/R group.
CONCLUSION: The activation of NF-κB plays an important role in the pathogenesis of lung injury induced by intestinal I/R through upregulating the neutrophil infiltration and lung ICAM-1 expression. PDTC as an inhibitor of NF-κB can prevent lung injury induced by intestinal I/R through inhibiting the activity of NF-κB.
- Citation: Tian XF, Yao JH, Li YH, Zhang XS, Feng BA, Yang CM, Zheng SS. Effect of nuclear factor kappa B on intercellular adhesion molecule-1 expression and neutrophil infiltration in lung injury induced by intestinal ischemia/reperfusion in rats. World J Gastroenterol 2006; 12(3): 388-392
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.388
Intestinal I/R is not necessarily limited to the intestine itself, but involves severe destuction of distant tissue. It is known that intestinal I/R is an important event in the pathogenesis of multi-system organ failure syndrome, especially acute respiratory distress syndrome which is the leading cause of death in critically ill patients[1-4]. The mechanism of lung injury induced by intestinal I/R is complex. Proinflammatory cytokines and chemokines exert their effects via a direct toxic action on target cells[5], ICAM-1 and neutrophil infiltration play an important role in lung injury induced by intestinal I/R[6]. However, recent studies showed that these proinflammatory mediators play a role in gene induction[7-9]. NF-κB upregulates most of these mediators[10-12], but it is not known how NF-κB is activated and ICAM-1 is expressed in lung injury induced by intestinal ischemia.
This study was to evaluate the role of NF-κB in the pathogenesis of lung injury induced by intestinal I/R and the effect of pyrrolidine dithiocarbamate (PDTC) on lung neutrophil infiltration and expression of ICAM-1.
Male Wistar rats (From Animal Center of Dalian Medical University, Dalian, China) weighing 200-240 g were used in this study. All rats had free access to standard laboratory chow and water in accordance with institutional animal care policies.
The rats were anesthetized with intraperitoneal administration of 10% chloral hydrate, 350 mg/kg, laparotomized and randomly divided into three experimental groups (n = 8 in each): sham operation group (control group) undergoing full surgical preparation including isolation of SMA without occlusion; I/R group: ischemia was induced for 1 h and reperfusion for 2 h after SMA was isolated and ischemia was occluded[13]; PDTC treatment group undergoing full surgical preparation including isolation of SMA with intraperitoneal administration of 2% PDTC 100 mg/kg 1 h before the operation. The rats in control and I/R groups were treated with an equal volume of normal saline solution. All animals were killed after 2 h of reperfusion. Blood samples were obtained for analysis. Lung tissues were harvested immediately for detection.
The harvested right middle lobe of the lung was fixed in 40 g/L formaldehyde. After being embedded in paraffin, 4-μm-thick sections were stained with hematoxylin and eosin for light microscopy. Pathological injury score was evaluated according to Chiu’s method[14]. BALF was collected according to the process of Cox[15] and centrifuged at 1 000 r/min for 10 min. The protein in the supernatant was measured using assay kit (Nanjing Jincheng Corp., China) following the manufacturer’s instructions and expressed as g/L.
The serum level of IL-6 was determined using an RIA kit (Radioimmunity Institute of PLA General Hospital, Beijing, China) according to the manufacturer’s instructions and expressed as ng/L.
The right base lobe of lung was harvested and immediately homogenized on ice in 5 volumes of normal saline. The homogenates were centrifuged at 1 200 r/min for 10 min. The malondialdehyde (MDA) and myeloperoxidase (MPO) content in the supernatants were measured using MDA and MPO assay kit (Nanjing Jincheng Corp., China) following the manufacturer’s instructions and expressed as nmol/mg and U/g, respectively.
Formalin-fixed and paraffin-embedded lung specimens were stained with SP immunohistochemistry technique for NF-κB and ICAM-1 detection. Experiments were performed following the manufacturer’s instructions. Five-micron sections were dewaxed in xylene, cultured in 3% hydrogen peroxide to eliminate intrinsic peroxidase and quenched in normal goat serum for 30 min, then incubated overnight at 4 ºC with polyclonal rabbit anti-rat NF-κB p65 and ICAM-1 antibody (NeoMarkers Corp. and Boster Corp., Ltd., respectively) against purified recombinant NF-κB or ICAM-1. Then anti-rabbit immunoglobulin and streptavidin conjugated to horseradish peroxides were added. Finally, 3,3’-diaminobenzidine was used for color development and hematoxylin was used for counter staining. The results were evaluated semi-quantitatively according to the percentage of positive cells in five high power fields at 400 multiple signal magnification.
Cellular plasma and nuclear protein were extracted from frozen lung tissue with protein extraction kit (Pierce, Meridian Road, Rockford, IL, USA) according to the manufacturer’s instructions. Protein concentrations were determined by Coomassie blue dye-binding assay (Nanjing Jincheng Corp, China). Samples were mixed with loading buffer and boiled for 5 min. Thirty micrograms of protein (nuclear protein for NF-κB p65, plasma protein for ICAM-1) was loaded into each lane of 10% SDS-PAGE gel electrophoresis at 100 V for 4 h. After electrophoresis, the proteins were electroblotted onto NC membranes (Millipore, Bedford, MA, USA) at 9 V for 30 min. Nonspecific binding was blocked by incubation in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T) and 5% skim milk. The transferred membranes were incubated overnight at 4 °C with rabbit polyclonal antibodies NF-κB p65 and ICAM-1 (NF-κB p65 at 1:1 000 dilution, ICAM-1 at 1:500 dilution) against rat in PBS-T containing 5% skim milk. After washing thrice in PBS-T, the membranes were incubated with anti-rabbit IgG (Zhongshan Bio., China) conjugated to horseradish peroxidase at a dilution 1:2 000 in PBS-T containing 5% skim milk for 1 h at 37 °C. After three additional washes with PBS-T, the signals were visualized by DAB assay kit (Maixin-bio, China) and analyzed with a gel imaging system (Kodak system EDAS120).
All data were expressed as mean ± SD. Statistical analysis was performed using F- and Q-tests. P < 0.05 was considered statistically significant.
The lung histological structure was normal in control group, while the lung tissues were obviously damaged with edema, hemorrhage, and inflammatory cell infiltration in I/R group. There was a significant difference between I/R and control group in pathological score (P < 0.01) and BALF content. After the administration of PDTC, the pathological score of lung injury and BALF content was improved significantly when compared to I/R group (P < 0.05, Table 1).
Compared to control group, serum IL-6 level was significantly increased in I/R group (P < 0.01, Table 2). Compared to I/R group, serum IL-6 level was significantly decreased in PDTC treatment group (P < 0.05).
Compared to control group, lung MDA and MPO significantly increased in I/R group (P < 0.01). Compared to I/R group, lung MDA and MPO significantly decreased in PDTC treatment group (P < 0.01, Table 3).
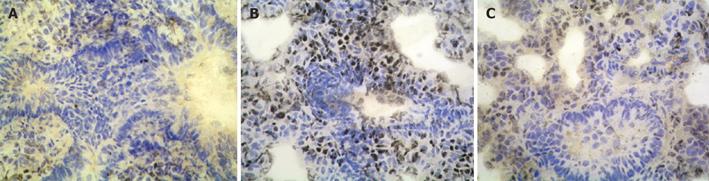
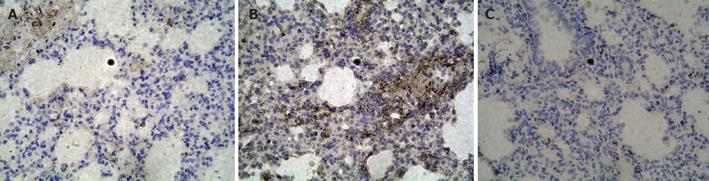
The expression of NF-κB p65 and ICAM-1 in control group showed light brown immunostaining in cytoplasm and no staining in the nuclei. The significant positive expressions of NF-κB p65 and ICAM-1 as strong brown staining in cytoplasm and nuclei were observed in I/R group (P < 0.01). Compared to I/R group, the positive rates of NF-κB p65 and ICAM-1 expression decreased significantly in PDTC group (P < 0.01, Figures 1 and 2).
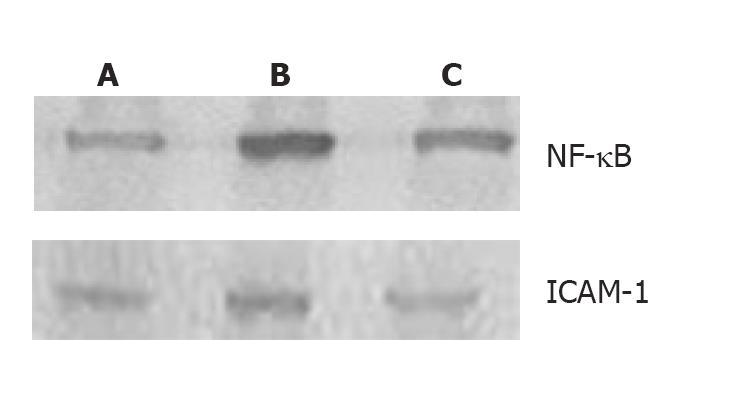
Western blot showed weak NF-κB p65 and ICAM-1 positive signals in the lungs of control group. In contrast, significant increase of NF-κB p65 and ICAM-1 protein expression was found in I/R group (P < 0.01). Compared to I/R group, the signals weakened significantly in PDTC group (P < 0.05, Figure 3 and Table 4).
Previous studies have identified many mediators involved in the pathogenesis of lung injury induced by intestinal I/R[16,17], which exert their effects via a direct toxic action on lung tissue. Recent studies showed that these mediators such as NO, ROS, TNF-α, and ICAM-1 can be regulated by NF-κB. NF-κB is a rapid response transcription factor, which is maintained in the cytoplasm and consists of two subunits of 50 and 65 ku bound to an inhibitor protein, I-κB. This phosphorylated inhibitor unit is tagged by ubiquitin for subsequent proteolysis, and then the free NF-κB complex is able to translocate into the nuclei where it transactivates target genes[18,19]. As a consequence, activated polymorphonuclear neutrophils (PMNs) and pro-inflammatory cytokines (TNF-α, ILs) are released into the systemic circulation, interact with the vascular endothelium of distant organs, primarily the lungs, contributing to the systemic inflammatory response[20]. ICAM-1 is a member of the immunoglobulin superfamily, which is inducible by NF-κB and inflammatory cytokines such as IL-1β and TNF-α. ICAM-1 might be upregulated to involve the adhesion and infiltration of leukocytes into the injured site[21]. MPO resides in PMNs and its activity reflects the level of accumulation of PMNs.
In our study, 1 h of intestinal ischemia followed by 2 h of reperfusion induced lung injury manifested as a significant increase of BALF content and pathological injury score as well as PMN infiltration. These changes were parallel to the level of lung NF-κB p65, suggesting that the activation of NF-κB is involved in the pathogenesis of lung injury induced by intestinal I/R. As a consequence, activated PMNs and pro-inflammatory cytokines (such as IL-6) are released into the systemic circulation, and interact with the vascular endothelium of organs. Endothelial adhesion molecules expressed on the surface of endothelial cells (such as ICAM-1) play a key role in neutrophil chemoattraction, adhesion and emigration from the vasculature to the tissue, contributing to the systemic inflammatory response and organ injury[22,23]. The production of ROS, such as lung MDA and MPO (an index of tissue neutrophil count) was observed in I/R group. NF-κB upregulates neutrophil infiltration and expression of ICAM-1. PDTC, as an antioxidant, is involved in its ability to inhibit NF-κB[24-26] via the stabilization of I-κB-α[27] or via the inhibition of the ubiquitin–proteasome pathway[28]. In our study, PDTC reduced the neutrophil infiltration, lung expression of ICAM-1 and MPO activity, which can prevent the development of lung injury.
In conclusion, activation of NF-κB plays an important role in the pathogenesis of lung injury induced by intestinal I/R by upregulating the neutrophil infiltration and lung ICAM-1 expression. PDTC can prevent lung injury induced by intestinal I/R by inhibiting the activity of NF-κB.
We thank Professors Sen Lu and Min Liu, Laboratory of Molecular Biology (State Administration of Traditional Chinese Medicine), Second Affiliated Hospital of Dalian Medical University for their technical assistance.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Nüssler NC, Müller AR, Weidenbach H, Vergopoulos A, Platz KP, Volk HD, Neuhaus P, Nussler AK. IL-10 increases tissue injury after selective intestinal ischemia/reperfusion. Ann Surg. 2003;238:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Pierro A, Eaton S. Intestinal ischemia reperfusion injury and multisystem organ failure. Semin Pediatr Surg. 2004;13:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Antonsson JB, Fiddian-Green RG. The role of the gut in shock and multiple system organ failure. Eur J Surg. 1991;157:3-12. [PubMed] |
| 4. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 641] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Hu XM, Lu Y, Yao SL. [Propofol reduces intercellular adhesion molecular-1 expression in lung injury following intestinal ischemia/reperfusion in rats]. Zhongguo Weizhongbing Jijiu Yixue. 2005;17:53-56. [PubMed] |
| 7. | Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4113] [Cited by in RCA: 4135] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 9. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3589] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 10. | Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1994;91:11641-11645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270:933-943. [PubMed] |
| 12. | Xu J, Xie J, Bao M, Li Z, Yang Z. NF-kappaB/I-kappaB pathway during ischemia reperfusion injury of rat liver. Chin Med J (Engl). 2003;116:1146-1149. [PubMed] |
| 13. | Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1426] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 15. | Cox G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol. 1996;271:L566-L571. [PubMed] |
| 16. | Galili Y, Ben-Abraham R, Weinbroum A, Marmur S, Iaina A, Volman Y, Peer G, Szold O, Soffer D, Klausner J. Methylene blue prevents pulmonary injury after intestinal ischemia-reperfusion. J Trauma. 1998;45:222-25; discussion 222-25;. [PubMed] |
| 17. | Chen ZB, Zheng SS, Yuan G, Ding CY, Zhang Y, Zhao XH, Ni LM. Effects of intestinal lymph on expression of neutrophil adhesion factors and lung injury after trauma-induced shock. World J Gastroenterol. 2004;10:3221-3224. [PubMed] |
| 18. | Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 274] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 737] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339-27342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 523] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Mollà M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, Piqué JM, Panés J. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003;57:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Ishii H, Ishibashi M, Takayama M, Nishida T, Yoshida M. The role of cytokine-induced neutrophil chemoattractant-1 in neutrophil-mediated remote lung injury after intestinal ischaemia/reperfusion in rats. Respirology. 2000;5:325-331. [PubMed] |
| 24. | Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402-G1414. [PubMed] |
| 25. | Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 689] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 26. | Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976-3983. [PubMed] |
| 27. | Virlos I, Mazzon E, Serraino I, Di Paola R, Genovese T, Britti D, Thiemerman C, Siriwardena A, Cuzzocrea S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock. 2003;20:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol. 2005;79:8014-8023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |