Published online Aug 7, 2006. doi: 10.3748/wjg.v12.i29.4727
Revised: August 28, 2005
Accepted: October 10, 2005
Published online: August 7, 2006
AIM: To test the feasibility of delivering a plasmid encoding IL-15 as a DNA vaccine adjuvant for improving the immune responses induced by hepatitis B virus core gene DNA vaccine.
METHODS: We used RT-PCR based strategies to develop IL-15 expression constructs. We first confirmed that the gene could be expressed in Escherichia coli due to the poor expression of IL-15. Then the bioactivity of IL-15 plasmid expression product was identified by CTLL-2 proliferation assay. One hundred micrograms of DNA from each of the IL-15 eukaryotic expressed plasmid and the recombinant plasmid harboring DNA encoding the 144 amino acids of the N-terminus of HBV core gene (abbreviated pHBc144) was used to co-immunize C57 BL/6 mice. The titer of anti-HBcIgG was detected by ELISA and the antigen-specific CD8+ T cells (CD8+IFN-γ+ T cells) were detected by intracellular cytokine staining at different time points.
RESULTS: After co-immunization by pIL-15 and pHBc144 DNA vaccine the antigen-specific CD8+ cells of mice increased gradually, the first peak of immune response appeared 14 d later, then the number of antigen-specific CD8+ Ts cells decreased gradually and maintained at a steady level in 3 mo. After boosting, the number of antigen-specific CD8+ T cells reached the second peak 10 d later with a double of the 1st peak, then the number of antigen-specific CD8+ T cells decreased slowly. IL-15 as a gene adjuvant had no significant effect on humoral immune responses induced by hepatitis B virus core gene DNA vaccine, but increased the memory antigen-specific CD8+ T cells induced by hepatitis B virus core gene DNA vaccine.
CONCLUSION: DNA vaccine constructed by HBc Ag 1-144 amino acid induces effective cell immunity, and cytokine plasmid-delivered IL-15 enhances the longevity of CD8+ T cells.
- Citation: Zhang W, Dong SF, Sun SH, Wang Y, Li GD, Qu D. Coimmunization with IL-15 plasmid enhances the longevity of CD8 T cells induced by DNA encoding hepatitis B virus core antigen. World J Gastroenterol 2006; 12(29): 4727-4735
- URL: https://www.wjgnet.com/1007-9327/full/v12/i29/4727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i29.4727
The final goal of a vaccine is to make body acquire long-term and effective protection. However the long-term and effective protection depends on the quality and quantity of memory T cells. IL-15 mRNA is constitutively expressed by various cells and tissues such as placenta, skeletal muscle, kidney, epithelial cells, synovial cells, and macrophages[1]. IL-15 is essential for CD8+ memory T-cell generation and maintenance, and is involved in the maintenance of antigen-specific memory CD8+ T cells. The number of memory-type (CD44highLy6C) CD8+ T cells increases significantly in the periphery lymphoid tissue of IL-15 Tg mice, producing IL-15, while the number of antigen-specific memory CD8+ T cells declines in IL-15- and IL-15R-deficient mice[2].
IL-15 has attracted great attention because of its important effect on memory CD8+ T cells[3-5]. This report describes the IL-15 mRNA extraction from human peripheral blood mononuclear cells (PBMCs) and the molecular cloning of IL-15. We demonstrated that recombinant IL-15 was expressed in Escherichia coli and showed bioactivity in COS cells. Our results suggest that IL-15 plasmid has effect on the memory phase of cellular immune response induced by HBc DNA vaccine, indicating that plasmid IL-15 functions as a candidate adjuvant and can be used in for vaccine or immunotherapeutic studies.
Female C57BL/6 mice at the age of 6-8 wk were purchased from Shanghai Sippr-Bk Company and allowed to acclimate for at least 2 wk prior to use. The mice were housed in laminar airflow cages with free access to food and water ad libitum. Analyses of specimens from those animals revealed no evidence of exposure to viral, bacterial or parasitic pathogens during these studies.
The HBcAg-expression plasmid pHBc144 was constructed by inserting a gene encoding hepatitis B core antigen N’ end 144 amino acid into a vector (pcDNA3, Invitrogen), driven by the cytomegalovirus (CMV) immediate early promoter. The secreted HBcAg could be detected in the supernatant of cell culture transiently transfected with pHBc144[6]. Anti-HBc could be detected in the serum of Balb/c mice, which were injected with pHBc144 DNA plasmid.
The HBV core Ag peptides were synthesized and generously provided by Dr. Rafi Ahmed (Emory University, GA)
PBMCs were isolated from peripheral blood of healthy volunteers by density gradient centrifugation over Ficoll. Fresh whole blood was drawn, heparinized and gently mixed with one volume of RPMI1640. The diluted blood was layered over Ficoll and centrifuged at 800 g, for 20 min at 4°C. PBMC were collected from the interface between the plasma and the density gradient solution. After being washed in PBS, PBMCs were resuspended in RPMI 1640 supplemented with 5 mg/mL LPS, 200 mmol/L L-guatmine, 10% FCS, 20 mmol/L Hepes, 100 U/mL penicillin, and 100 μg/mL streptomycin, at a final cell concentration of 5 ×106/mL. Then the PBMCs were cultured in 24-well plates for 6 h.
RNA extraction as well as RT-PCR analysis were performed as previously described[7]. Briefly, total RNA was purified using Trizol (Invitrogen Life Technologies) following the manufacturer’s instructions. A 4 μg aliquot of total cellular RNA was reverse transcribed using random hexanucleotide primers and the Superscript II preamplification system (Invitrogen Life Technologies). One-tenth of the cDNA obtained was amplified in a 20-pl reaction, using 1 U of Pwo DNA Polymerase ExpandTM High Fidelity PCR System (Boehringer Mannheim) and the reagents provided with the preamplification system. Thirty-five cycles of PCR amplification were performed at 94°C for 2 min, at 60°C for 1 min and at 72°C for 1 min; followed by a final extension at 72°C for 7 min. Ten microliters of the reaction products was analyzed on 1% agarose gel. The primer sequences are sense: 5’-GCGGAATTCATGAGAATTTCGAAACCACATTTGAG-3’; antisense: 5’-GCGCTCGAGAATCAATTGCAATCAAGAAGTGT-3’. The amplified cDNA was ultimately digested with EcoR I and Xho I and cloned into pcDNA3 and pET2c. The sequences of the IL-15 DNA insert were verified by sequencing with the dideoxy chain termination method (Bioasia Co., Shanghai). Sequences were analyzed by Vector NTI Suite software package (InfoMax).
Escherichia coli strain BL21(DE3) (Novagen) was transformed by the pET2c-IL-15 plasmid. The detailed protocol for production of fusion proteins was as follows. E.coli cells were grown to absorbance 0.6 at 600 nm, then expression was induced with 0.4 mmol/L isopropyl-D-thiogalac-toside for 4 h at 37°C. The bacterial cells were harvested, the cell pellet was suspended in 50 mL MTPBS lysis buffer (150 mmol/L NaCl, 16 mmol/L NaH2PO4, 4 mmol/L Na2HPO4, pH 7.3, 1% TritonX-100) per liter of culture volume. The cells were sonicated three times, 10 s each time (Sinifer cell disrupter B-30; Branson Sonic Power), and centrifuged at 15 000 g for 10 min at 4°C. The supernatant was collected for SDS-PAGE and Western blotting.
E.coli cells were cultured for an indicated period of time, lysed by adding 2X Laemmli SDS sample buffer and boiled for 5 min in a water bath. The proteins were resolved by 15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immun-blot PVDF; Bio-Rad). Non-specific binding sites were blocked by incubating the blot in blocking buffer (5% nonfat dry milk in wash buffer) for 1 h at room temperature. The blot was washed three times (5 min each time) with wash buffer (0.5% Tween 20, 200 mmol/L NaCl, and 50 mmol/L Tris, pH 7.5), and incubated with the appropriate primary Ab overnight at 4°C. The blot was then washed three times (5 min each time) and incubated with the corresponding secondary Ab-HRP conjugate for 1 h at room temperature. The blot was washed three times again (5 min each time), and proteins were detected.
For CTLL-2 bioassays, COS cells were transfected with 30 μg of IL-15 expression constructs following a standard calcium-phosphate-mediated transfection protocol[7]. For these studies roughly 2-3 × 105 adherent COS cells/well were transfected in six-well 35-mm plates. After 24 h, the medium was replaced with fresh complete DMEM, supernatants were harvested 72 h after the transfection for assay.
Aliquots (100 μL) of the supernatant and serial two-fold dilution were added to 96-well microtiter plates containing 1 × 105 CTLL-2 cells in a final volume of 200 μL. The cultures were incubated for 18 h at 37°C and pulse-labeled for another 6 h with 0.5 μCi [3H] thymidine. Thymidine incorporation was quantified by liquid scintillation spectrometry[8] (BD). A standard curve was plotted from proliferation induced by standard murine rIL-2.
Plasmids were amplified in Escherichia coli and plasmid DNA was purified by using anion exchange column (Endofree plasmid purification kit, Qiagen, Chatworth, Calif) according to the manufacturer’s instructions. The plasmid DNA was dissolved in sterile physiological saline, and the final concentration was 1 μg/mL.
C57BL/6(H-2b)mice were vaccinated at the age of 6-8 wk by injection of DNA in 0.9% saline (w/v), into the quadriceps of each hind limb as previously described[9]. Injection volume was 50 μL per leg and total DNA dose was 50 μg per leg. On d 30, all mice were booster-immunized with the same DNA. On d 0, 8, 14, 30, 90 after priming and d 90 after boostering, blood samples were collected from each mouse by orbital bleeding (Figure 1). Serum was separated from each blood sample and used for measurement of the anti-HBcAb titer. On the other hand, mouse blood was obtained using heparin as an anticoagulant. The blood was diluted with RPMI 1640. PBMCs were obtained by density gradient centrifugation over HISTOPAQUE-1083 (Sigma), washed three times with RPMI 1640 and counted afterward. The spleens were removed for the measurement of cytokine production.
Anti-HBc antibody was titrated with ELISA kit (Shanghai Infectious Diseases’ Hospital). Each serum sample was made in a series of three-fold dilution. Goat anti-mouse IgG-HRP conjugate (The Binding Site Limited Co, England) was used as the secondary antibody and O-pheylenediamine (OPD) was used as a substrate. The plates were read with Titerek Multiskan MCC/340 at A450.The wells with an absorbance of ≥ 0.1 (above background, 0.020) were scored as positive.
For intracellular cytokine detection of IFN-γ, PBMCs and single suspension of splenocytes were plated in a 96-well plate (Costar) at a density of 1 × 106 cells/well in a final volume of 200 μL. Cells were stimulated with peptides for 5 h. Brefeldin A (final concentration 2 μg/mL) was added to the cultures during stimulation. At the end of the incubation, the cells were washed twice and stained for 30 min at 4°C with phycoerythrin (PE)-conjugated anti-mouse CD8 (BD Bioscience) in FACS buffer [2% FCS, 0.1% sodium azide in phosphate buffered saline (PBS)]. Cells were collected, washed and incubated in permeabilization buffer for 20 min on ice. The permeabilized cells were washed and stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IFN-γ (BD PharMingen, San Diego, CA) for 30 min. Cells were washed, fixed with 2% (w/v) paraformaldehyde, and acquired with a FACSCalibur flow cytometer (Becton Dickinson, Boston, MA). Forward and side scatters were collected on a linear scale, while the PE and FITC signals were collected on a 4-decade log scale. Overlaps of emission spectra were electronically compensated. The debris were eliminated using the threshold on forward scatter and 100 000 events were acquired with CellQuest software (BD, Biosciences).
Statistical analyses of data were performed using SPSS 10.0 for windows (SPSS, Inc., Chicago, IL).
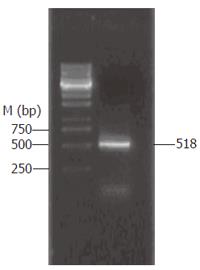
PBMCs were isolated from peripheral blood of healthy volunteers by density gradient centrifugation over Ficoll. A total of 5 × 105 PBMCs were transferred into 24-well plates and cultured for 6 h with 5 μg/mL LPS. RNA extraction and RT-PCR were performed (Figure 2). The complete human IL-15 cDNA (GenBank accession No. U14407) was 518 bp long and was comprised of 146 bp signal peptide (IL-15sp) and a IL-15 mature peptide gene sequence (IL-15mp).
Amplified cDNA was ultimately digested with EcoR I and Xho I and cloned into pcDNA3. The sequences of IL-15 DNA insert were confirmed and this plasmid was named pIL-15.
For generation of the IL-15 prokaryotic expression construct, the IL-15 mp coding sequence was PCR amplified from pIL-15. The resulting fragments were digested with EcoR I and Xho I and cloned into pET2c named pET-IL-15 (data not shown) .
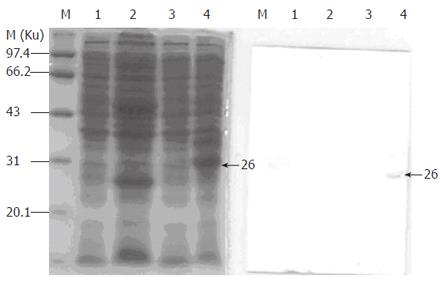
Escherichia coli strain BL21(DE3) (Novagen) was transformed by the pET-IL-15 plasmid. After induction by IPTG and temperature, the supernatant of bacteria lysates was taken for SDS-PAGE and Western blotting. IL-15 fragments could be expressed in pET-32c vector and its molecular weight was Mr 26 000 (Figure 3).
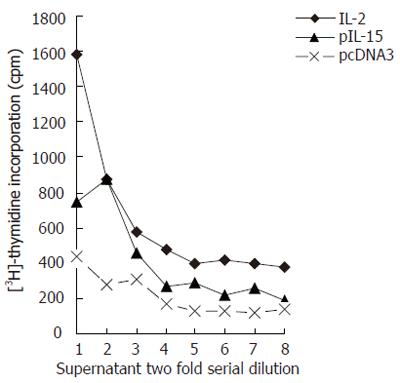
The IL-15 was cloned into the pcDNA3 vector with CMV promoter (Invitrogen) and transfected into COS cells. Forty-eight hours after transfection, the COS supernatants were collected and subjected to CTLL-2 proliferation assay. CTLL-2 cell 3H-TdR cpm was related to the dilution fold of the COS supernatant. When the dilution fold of transfection supernatant increased, the incorporation of 3H-TdR cpm decreased. The incorporation 3H-TdR cpm of pIL-15-transfected COS supernatant was higher than that of vector-tansfected COS supernatant (Figure 4). So we could detect IL-15 activity in the supernatants of COS cells transfected with pIL-15.
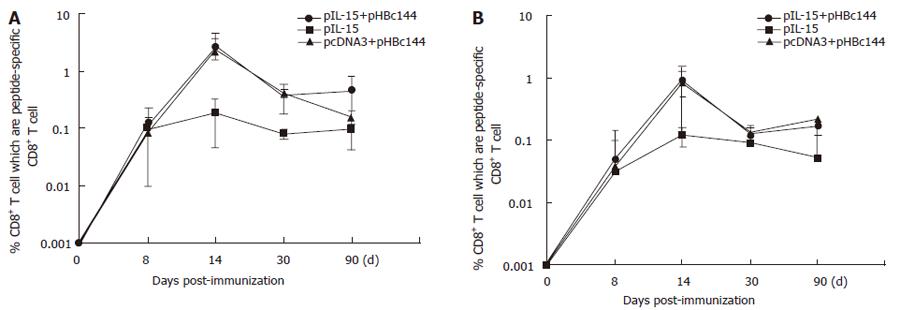
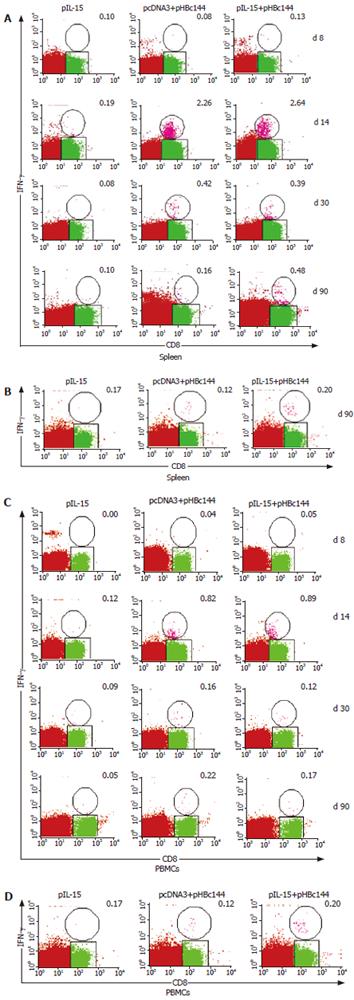
Both primary and memory antigen-experienced CD8+ T cells rapidly upregulated cytokine production after encountering cells displaying their cognate peptide ligand in association with a major histocompatibility complex class I molecule. We used ICCS to identify cytokine-producing HBc-specific CD8+ memory T cells directly ex vivo after DNA inoculation prior to this study, but it was unclear how quickly these responses developed when IL-15 as a gene adjuvant was coinjected with HBc DNA vaccine. Therefore, we used ICCS to directly enumerate antigen-specific effector CD8+ T cells in the spleens of mice at various time points after DNA immunization (pHBc144 and/or pIL-15). The average percentage of peptide-specific CD8+ T cells detected in three mice analyzed at each time point for each mode of immunization is shown in Figure 5A.
Seven days after-immunization, antigen specific CD8+ T cells were present at a frequency of less than 0.1% in all of the vaccinated mice (both pHBc144 + pcDNA3 group and pHBc144 + pIL-15 groups), and therefore accurate analysis of IFN-γ production was not possible (Figure 5A). By d 14, 2.64% ± 1.07% of the HBcAg specific CD8+ T cells primed by pHBc144 + pIL-15 DNA vaccination secreted IFN-γ, and at 30 d after-immunization, 0.39% ± 0.21% of the antigen-specific cells were positive. A similar transition was observed in CD8+ T cells primed by pHBc144 + pcDNA3 DNA vaccination (2.26% ± 2.50% on d 14 and 0.42% ± 0.06% on d 30). Thus, the cytokine expression patterns of HBcAg-specific T cells were similar regardless of the pIL-15 immunization.
These proportions decreased gradually, by d 90 post-priming, 0.48% ± 0.36% of the antigen-specific CD8+ T cells induced by pHBc144 in pIL-15 co-immunized group secreted IFN-γ, while 0.16% ± 0.04% of the antigen-specific CD8+ T cells in pHBc144 and pcDNA3 co-immunized group were IFN-γ+CD8+ T cells (Figure 6).
After being boosted, the HBc Ag specific T cells expanded rapidly, and by d 90 post-boost the specific memory cells could still be detected, sustaining a higher level than that for the first response. On d 90 after boosting, 0.28% ± 0.04% of the antigen-specific CD8+ T cells induced by pHBc144 in pIL-15 co-immunized group secreted IFN-γ, while 0.11% ± 0.04% of the antigen-specific CD8+ T cells in pHBc144 and pcDNA3 co-immunized group were IFN-γ+ CD8+ T cells (Figures 6A and B ).
For examining the kinetics and distribution of antigen specific CD8+ T-cells in the non-lymphoid tissue induced by DNA vaccination, we separated the lymphocytes from blood. The kinetics of HBcAg-specific CD8+ T cells in the circulation stimulated with pHBc144 showed a similar pattern with that of the spleen (Figure 5). However the number of HBcAg-specific CD8+ T cells in PBMCs was lower than that in the spleen at the same time point. Eight days after DNA vaccination, HBcAg-specific CD8+ T cells were detectable. By d 14 the number of IFN-γ+ CD8+ T cells in PBMCs reached the peak and then dropped gradually. These proportions decreased gradually until d 90 post-priming, 0.17% ± 0.05% of the antigen-specific CD8+ T cells induced by pHBc144 in pIL-15 co-immunized group secreted IFN-γ, while 0.22% ± 0.01% antigen-specific CD8+ T cells in pHBc144 and pcDNA3 co-immunized group were IFN-γ+ CD8+ T cells.
On d 90, after boosting, 0.20% ± 0.13% of the antigen-specific CD8+ T cells induced by pHBc144 and pIL-15 co-immunized group secreted IFN-γ, while 0.12% ± 0.13% antigen-specific CD8+ T cells in pHBc144 and pcDNA3 co-immunized group were IFN-γ+ CD8+ T cells (Figure 5B, Figure 6 C and D ). The antigen-specific memory cells still could be detected directly ex vivo in the circulation on d 90 post-boost (Figure 5B, Figure 6D).
At the same time point, we checked the total CD4+ and CD8+ cell percentage (Table 1), IL-15 had no big effect on the ratio of CD4+ and CD8+.
| Group | d 8 | d 14 | d 30 | d 90 | booster d 90 | |||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| A Spleen | ||||||||||
| pIL-15+pHBc144 | 13.35 ± 1.00 | 8.31 ± 2.08 | 17.89 ± 0.83 | 15.15 ± 0.88 | 22.02 ± 2.12 | 12.63 ± 0.51 | 20.23 ± 3.07 | 23.41 ± 0.65 | 11.76 ± 2.36 | 12.10 ± 0.59 |
| pcDNA3+pHBc144 | 14.08 ± 1.32 | 8.24 ± 1.08 | 16.69 ± 2.21 | 14.75 ± 0.87 | 19.56 ± 1.23 | 14.58 ± 0.98 | 19.77 ± 3.61 | 22.37 ± 1.53 | 12.03 ± 2.50 | 13.43 ± 1.93 |
| pIL-15 | 14.43 | 10.12 | 14.69 ± 2.25 | 10.31 ± 1.94 | 19.76 ± 1.41 | 13.38 ± 1.53 | 19.04 ± 1.75 | 15.72 ± 3.08 | 11.08 ± 0.21 | 12.76 ± 2.55 |
| B PBMC | ||||||||||
| pIL-15+pHBc144 | 13.05 ± 1.75 | 7.42 ± 1.41 | 15.93 ± 2.95 | 10.67 ± 1.87 | 19.10 ± 3.70 | 10.06 ± 0.47 | 16.94 ± 1.19 | 8.61 ± 1.20 | 8.66 ± 0.13 | 12.78 ± 1.76 |
| pcDNA3+pHBc144 | 15.49 ± 4.77 | 8.35 ± 1.84 | 18.30 ± 3.56 | 12.24 ± 1.27 | 22.97 ±3.17 | 12.79 ± 4.39 | 7.50 ± 0.58 | 7.18 ± 2.93 | 10.09 ± 5.19 | 16.20 ± 1.48 |
| pcDNA3+pHBc144 | 14.85 | 8.92 | 17.98 ± 3.20 | 7.68 ± 3.46 | 21.70 ± 5.44 | 12.51 ± 1.16 | 12.58 ± 0.66 | 9.43 ± 1.36 | 8.36 ± 3.53 | 10.34 ± 2.09 |
The HBcAg specific humoral immune response induced by pHBc144 was detected by ELISA for anti-HBc antibody in serum. The plates were read with Titerek Multiskan MCC/340 at A450.The wells with an absorbance of ≥0.1 (above background, 0.020) were scored as positive.
Four weeks after the first pHBc144 immunization, all mice underwent anti-HBc seroconversion. The mean titer of anti-HBc IgG in pHBc144 + pIL-15 group reached 29.81 ± 20.9 on d 30. After being boosted, the mice produced a higher titer of anti-HBc IgG (29.81 ± 20.9) than that of the primary response, and anti-HBc maintained at that level (29.81 ± 20.9) on d 90. The titer of anti-HBc IgG2a in pHBc144 + pIL-15 group (29.81 ± 20.9) was slightly higher than that of pHBc144 + pcDNA3 group (28.22 ± 20.9), but there was no significant difference (P > 0.05).
IL-15 plays a pivotal role in the development, survival, and activation of NK cells, NK-T cells and cytokine release various subsets of T and B cells[10-12]. IL-15 maintains the homeostasis of memory phenotype CD8+ T cells[13]. The IL-15 response to infectious agents, such as viruses and other intracellular organisms, may represent a critical element in the host defense against these pathogens. Optimized IL-15 in combin with HIV-1gag DNA constructs results in a significant enhancement of Ag-specific CD8+ T cell proliferation and IFN-gamma secretion, and strong induction of long-lived CD8+ T cell responses[14]. pDNA encoding IL-15 is capable of elevating survival rates of HSV-1-infected mice when coinjected with 1 μg of gB pDNA[15]. IL-15 has been reported to have a profound effect on the augmentation of CD8+ T cell response against murine T. gondii infection[16]. Toxoplasma-specific CD8+ T cell immunity in mice is depleted over time, but can be rescued by IL-15 treatment. These findings suggest that IL-15 may be useful as an immune adjuvant given with vaccination to enhance its biologic efficacy.
IL-15 is predominantly regulated posttranscriptionally at the level of translation and translocation. Although Northern blot analysis can reveal widespread constitutive expression of IL-15 mRNA in a variety of tissues such as placenta, skeletal muscle, kidney, lung, heart, fibroblasts, epithelial cells, and monocytes, it is difficult to demonstrate IL-15 in supernatants of many cells that express such mRNA. This discordance between IL-15 mRNA expression and IL-15 protein production is regulated by multiple elements including 12 upstream AUGs of the 50 UTR, a 48-aa signal peptide, and the C-terminus of the mature protein. The IL-15 signal peptide is an important factor in the negative regulation of IL-15 protein expression. There are two alternative leader peptides, one with 48 aa and the other with 21 aa[17]. IL-15 associated with a short 21-aa signal peptide is not secreted but stored intracellularly, appearing in nuclear and cytoplasmic components[18]. The long 48-aa isoform of the signal peptide might function as a negative regulator of IL-15 generation. The total quantity of IL-15 generated (the sum of IL-15 retained within the cells) increases 17- to 20-fold when the IL-15 signal peptide is replaced by that of IL-2. Parallel studies showed that the quantity of IL-2 secreted is reduced 40- to 50-fold when COS cells are transfected with the reciprocal construct in which the IL-2 signal peptide is replaced with that of IL-15. Furthermore, after the IL-15 signal peptide is replaced with that of CD33 [19], translation and secretion increase, supporting the view that IL-15 expression is controlled posttranscriptionally at the level of translation and secretion.
We cloned the classical 48-aa signal peptide isoform of IL-15 (LSP-IL-15). When COS cells were transfected with the wild-type construct[19], the quantity of IL-15 protein was very low (360 pg per 200 000 cells). Moreover, in most cases it was difficult to demonstrate IL-15 in the culture supernatants. We could not detect IL-15 protein in culture supernatants by Western blot after transfecting COS cells with our construct (data not shown). We therefore subcloned the LSP-IL-15 cDNA into expression vector and let it translate in E coli. Western blot analysis showed a Mr 26 000 fusion protein, which was identified with NCBI sequence.
The IL-15 associated with the long 48-aa signal peptide enters the endoplasmic reticulum where it was glycosylated. Nevertheless, IL-15 is secreted after trafficking through the Golgi, yielding a cytokine with a fully processed signal peptide. We measured the biological function of the construct by detecting the IL-15 in the supernatant of transfection.
Meazza et al[20] and Onu et al[17] found that IL-15 is not secreted when IL-15 SP is employed. Nevertheless, Waldmann et al[21] assessed cultures from COS cells transfected with the wild-type IL-15 coding sequence at 120 h and found that at least 90% of the IL-15 protein (though at low levels) can be identified in the culture supernatants, and 10% is retained within the cells, as evaluated by sensitive ELISA assays. Our result is consistent with that of Waldmann et al. Although in COS cells transfected with IL-15 expression construct, the levels of IL-15 protein synthesized and secreted were very low (3 logs less than those obtained with a comparable IL-2 construct), LSP-IL-15 could secrete in the supernatant of COS cells transfected with IL-15 expression construct and have bioactivity, and can induce CTLL-2 cell proliferation. This difference between the different studies may be due to the fact that the CTLL-2 strain cannot respond to IL-15.
To determine if the IL-15 plasmid has many effects on the humoral and cellular immune response, especially CD8+ T cell response induced by DNA vaccination, splenocytes and PBMCs from the pIL-15 and HBcAg DNA vaccine co-immunized mice were evaluated for IFN-γ expression on antigen-specific CD8+ T cells and anti-HBc IgG in serum was detected at each time point. We selected them for the DNA plasmid format because they are inexpensive, noninfectious and can be readily optimized to enhance immunogenicity and protective efficacy. Furthermore, DNA vaccines can induce robust and durable CD8+ T cell responses, and can be used to deliver a large number of CTL epitopes[22]. Our results showed that pIL-15 had no effect on anti-HBc IgG induced by pHBc144. Although antigen-specific CD8+ T cells reached a peak on d 14 after priming, pIL-15 had no effect on antigen-specific CD8+ T cells in splenocytes or PBMCs induced by HBcAg DNA vaccine. pIL-15 could increase the percentage of antigen-specific CD8+ T cells induced by HBcAg DNA vaccine on d 90 after priming and boosting. These two time points belonged to memory phase of immune response.
The existence of the basic effector Th1 and Th2 subsets is now well accepted, and used to plan therapeutic and vaccine strategies. Th1 cells produce interlukin 2 (IL-2), interferon γ and lymphotoxin (LT), whereas Th2 cells produce IL-4, IL-5, IL-6, IL-9 and IL-13[23]. The function of Th1 and Th2 cells correlates well with their distinctive cytokines. Th1 cells are involved in cell-mediated inflammatory reactions. Several Th1 cytokines activate cytotoxic and inflammatory functions, while Th1 clones induce delayed-type hypersensitivity (DTH) reactions; and IFN-γ is commonly expressed at sites of DTH reactions Th2 cytokines promote antibody production, particularly IgE responses, and also enhance eosinophil proliferation and function. Accordingly, Th2 cytokines are commonly found in association with strong antibody and allergic responses. Unfortunately, we did not evaluate the effect of IL-15 on antigen specific Th1 cells induced by HBc DNA vaccine. But our results showed that IL-15 had no effect on the ratio of CD4+ and CD8+.
Chronic HBV infection is one of the most common infectious diseases worldwide and leads to a high morbidity and mortality due to the development of liver cirrhosis and cancer. Virus persistence is believed to be due to an insufficient antiviral Th cell and CTL response of the host[24]. Effective HBV-specific CD8+ T cell responses have been proven to inhibit virus replication independently of liver damage[25,26].
During HBV replication, the nucleocapsid or core particle recruits into the pregenomic RNA and viral reverse transcriptase, and provides the template on which the surface envelope components assemble. Recombinant Mr 21 000 (P21) core protein self-assembles into 28-nm particles representing the native hepatitis B core Ag (HBc Ag)[27]. Since it plays a central role in nucleocapsid formation and pregenomic viral RNA packaging, HBV core antigen represents another interesting target for DNA-based vaccine, although less data are available on DNA immunization to core antigen protein compared to the envelope protein. DNA-based immunization of mice to the HBV core has been shown to efficiently prime specific antibody and cytotoxic-T-lymphocyte responses[28].
Interferon is one of the most effective drugs for chronic HBV infection[29]. Experiments with HBV transgenic mice suggest that intrahepatic secretion of antiviral cytokines, such as IFN-γ and TNF-α by CTLs and Kupffer cells can interrupt the HBV life cycle without lysis of infected hepatocytes. The rapid reduction of HBV-DNA before hepatocellular damage in acute infections in chimpanzees and humans suggests that a similar non-cytolytic mechanism is, at least in part, responsible for inhibition of viral replication during natural infection[30].
In conclusion, DNA vaccine constructed by HBc Ag 1-144 amino acid induces effective cell immunity, cytokine plasmid-delivered IL-15 enhances CD8+ T cell longevity, IL-15 is a possible candidate adjuvant for HBV DNA vaccine.
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
| 1. | Yajima T, Nishimura H, Sad S, Shen H, Kuwano H, Yoshikai Y. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J Immunol. 2005;174:3590-3597. [PubMed] |
| 2. | Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 683] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y. Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guérin vaccination. Infect Immun. 2003;71:6045-6048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Toka FN, Rouse BT. Mucosal application of plasmid-encoded IL-15 sustains a highly protective anti-Herpes simplex virus immunity. J Leukoc Biol. 2005;78:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Lazarevic V, Yankura DJ, DiVito SJ, Flynn JL. Induction of Mycobacterium tuberculosis-specific primary and secondary T-cell responses in interleukin-15-deficient mice. Infect Immun. 2005;73:2910-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Yang L, Liu J, Kong YY, Wang Y, Li GD. DNA vaccine of the fusion gene of the core region of HBV and HCV. Zhongguo Kexue. 1999;29:246-252. |
| 7. | Sambrook J, Russell DW. Molecular Cloning: a laboratory manual. Third Edition. New York: Cold Spring harbor laboratory Press 2001; 14-20. |
| 8. | Mire-Sluis AR, Thorpe R. Laboratory protocols for the quantitation of cytokines by bioassay using cytokine responsive cell lines. J Immunol Methods. 1998;211:199-210. [PubMed] |
| 9. | Lu M, Isogawa M, Xu Y, Hilken G. Immunization with the gene expressing woodchuck hepatitis virus nucleocapsid protein fused to cytotoxic-T-lymphocyte-associated antigen 4 leads to enhanced specific immune responses in mice and woodchucks. J Virol. 2005;79:6368-6376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Yajima T, Nishimura H, Ishimitsu R, Watase T, Busch DH, Pamer EG, Kuwano H, Yoshikai Y. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198-1203. [PubMed] |
| 11. | Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23-35. [PubMed] |
| 13. | Klonowski KD, Lefrançois L. The CD8 memory T cell subsystem: integration of homeostatic signaling during migration. Semin Immunol. 2005;17:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112-123. [PubMed] |
| 15. | Cui FD, Asada H, Jin ML, Kishida T, Shin-Ya M, Nakaya T, Kita M, Ishii M, Iwai M, Okanoue T. Cytokine genetic adjuvant facilitates prophylactic intravascular DNA vaccine against acute and latent herpes simplex virus infection in mice. Gene Ther. 2005;12:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Khan IA, Moretto M, Wei XQ, Williams M, Schwartzman JD, Liew FY. Treatment with soluble interleukin-15Ralpha exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J Exp Med. 2002;195:1463-1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Onu A, Pohl T, Krause H, Bulfone-Paus S. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J Immunol. 1997;158:255-262. [PubMed] |
| 18. | Gaggero A, Azzarone B, Andrei C, Mishal Z, Meazza R, Zappia E, Rubartelli A, Ferrini S. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur J Immunol. 1999;29:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5' untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418-4426. [PubMed] |
| 20. | Meazza R, Gaggero A, Neglia F, Basso S, Sforzini S, Pereno R, Azzarone B, Ferrini S. Expression of two interleukin-15 mRNA isoforms in human tumors does not correlate with secretion: role of different signal peptides. Eur J Immunol. 1997;27:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, Waldmann TA. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci USA. 1997;94:14444-14449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Wilson CC, McKinney D, Anders M, MaWhinney S, Forster J, Crimi C, Southwood S, Sette A, Chesnut R, Newman MJ. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J Immunol. 2003;171:5611-5623. [PubMed] |
| 23. | Rajcáni J, Mosko T, Rezuchová I. Current developments in viral DNA vaccines: shall they solve the unsolved. Rev Med Virol. 2005;15:303-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Zheng BJ, Zhou J, Qu D, Siu KL, Lam TW, Lo HY, Lee SS, Wen YM. Selective functional deficit in dendritic cell--T cell interaction is a crucial mechanism in chronic hepatitis B virus infection. J Viral Hepat. 2004;11:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Wu Y, Zhang J, Chen S, Chen A, Wang L, Li J, Zhao T, Zou L, Tang Y, Tingrong L. Frequencies of epitope-specific cytotoxic T lymphocytes in active chronic viral hepatitis B infection by using MHC class I peptide tetramers. Immunol Lett. 2004;92:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Hacker HJ, Deres K, Mildenberger M, Schröder CH. Antivirals interacting with hepatitis B virus core protein and core mutations may misdirect capsid assembly in a similar fashion. Biochem Pharmacol. 2003;66:2273-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Xu W, Chu Y, Zhang R, Xu H, Wang Y, Xiong S. Endoplasmic reticulum targeting sequence enhances HBV-specific cytotoxic T lymphocytes induced by a CTL epitope-based DNA vaccine. Virology. 2005;334:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Intern Med. 2005;142:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Tang TJ, Kwekkeboom J, Laman JD, Niesters HG, Zondervan PE, de Man RA, Schalm SW, Janssen HL. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |