Published online Aug 7, 2006. doi: 10.3748/wjg.v12.i29.4652
Revised: May 28, 2005
Accepted: February 26, 2006
Published online: August 7, 2006
AIM: To clarify the safety and feasibility of hepatectomy for huge hepatocellular carcinoma (HCC).
METHODS: A total of 4765 patients with HCC operated at Tongji Hospital were retrospectively studied, of them, 780 patients had huge HCC (10 cm or more in diameter). Hepatectomy was carried out on 634 patients (81.2%). The majority of the liver resection were major resections, and combined resection of the adjacent organs or structures was common (17.2%). The liver resection was combined with portal vein thrombectomy in 139 patients (21.9%).
RESULTS: Postoperative complications were common (26.8%) and required another laparotomy to prevent the complications in 5 patients (0.8%). The 30-d mortality was 2.2%. The main causes of postoperative deaths were liver failure (n = 9), postoperative bleeding (n = 4) and septic complication (n = 1). The 3-, 5- and 10-year survival rates after liver resection were 35.1%, 18.2% and 3.5%, respectively.
CONCLUSION: Hepatectomy for huge HCC is safe and effective. It should be used to treat patients with low surgical risks and resectable tumours.
- Citation: Chen XP, Qiu FZ, Wu ZD, Zhang BX. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol 2006; 12(29): 4652-4655
- URL: https://www.wjgnet.com/1007-9327/full/v12/i29/4652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i29.4652
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, with an annual occurrence of at least one million new cases[1-3].
Hepatic resection is the mainstay of treatment for resectable tumours because of the proven impact of adequate tumor removal on prognosis. Recent improvements in perioperative management have made partial hepatectomy safe.
Growing indications are related to the resection extent and the approach of surgery in patients with HCC, and the maximum prognosis benefit can be combined with optimal safety. Therefore, surgeons should balance the benefits of oncological clearance against the risks of inadequate parenchymal preservation after liver resection.
A non-cirrhotic liver can tolerate up to 80% liver resection. The regenerative capacity of the liver enables a functional compensation within a few weeks and regeneration to roughly 75% of the preoperative liver volume within 1 year. Such a favourable outcome, however, cannot be extrapolated to cirrhotic liver resection which is commonly carried out for HCC.
Hepatectomy for huge HCC is associated with a higher operative morbidity and mortality because of the extent of liver resection, and a histological margin of over 1 cm is usually recommended to cut down on intrahepatic tumor recurrence[4-6]. Of the many prognostic factors associated with long-term survival, the size of tumor has a positive association after ‘curative’ resection for HCC[6-10]. The aim of this retrospective study was to find out whether liver resection for huge HCC (tumor bigger than 10 cm in diameter) is efficacious and safe.
A retrospective study was done on 4765 patients with HCC who were treated surgically at the Tongji Hospital, Wuhan, China from January 1972 to December 2002, and 780 (16.8%) patients had huge HCC over 10 cm in diameter. Liver resection was carried out on 634 patietns (81.2%).
The criteria for liver resection for huge HCC with curative intention were good general condition of the patient, good cardiopulmonary and renal functions, Pugh-Child’s A and B, normal indocyanine green test, tumor confined to one lobe of the liver, and no extrahepatic spread of disease with the exception of local invasion to adjacent organs.
Liver resection for huge liver tumours was performed as previously described[11,12]. In brief, we routinely used the Pringle’s manoeuvre to occlude the hepatic inflow. We divided the porta hepatis pedicle supplying the side of the liver to be resected as described by Launois[13]. We then transected the liver parenchyma using finger-fracture technique with a haemostat along the plane of transection. The hepatic vein draining the part of the liver to be resected was ligated and divided intrahepatically. Then the portion of the liver to be resected by division of the ligaments was mobilized. This method of liver resection prevented laceration of the major vessels during mobilization of the liver, avoided rupturing of the huge tumor due to excessive traction, and excessive bleeding from the venous collaterals due to cirrhosis and portal hypertension by leaving mobilization of the liver near the end of liver resection. Oncological principles of ligating the vessels prior to manipulation of tumor were followed to diminish shedding of tumor cells into the blood stream.
Of the 780 patients with huge HCC of over 10 cm in diameter treated surgically, 634 (81.2%) underwent liver resection. The remaining 146 patients had unresectable tumors at laparotomy. The reasons for unresectability were: tumours too extensively involving the liver (n = 27), multiple secondary tumors (n = 56), severe liver cirrhosis (n = 44), and tumor infiltration into major vascular structures including the main portal vein, common hepatic artery or the hepatic veins (n = 19). These patients with unresectable HCC were treated with intraoperative transhepatic or transportal vein chemotherapy, or with chemotherapy via a totally implantable drug infusion system (n = 57), intraoperative microwave coagulation (n = 36), intraoperative cryotherapy (n = 45), or a biopsy of the liver tumor (n = 8).
The demographic data of the 634 patients who undergoing liver resection are shown in Table 1. Majority of the patients underwent major liver resection. The terminology of the liver resection used was included according to the recommendation by the International Hepato-Pancreato-Biliary Association. Combined resection of adjacent organs involving the tumor was common. A significant proportion of patient had tumor thrombi in the portal vein (Table 2).
| Sex (male:female) | 563:71 |
| Age (yr)1 | 39 ± 9.5 |
| Total bilirubin (μmol/L)1 | 12.5 ± 2.8 |
| Alanine aminotransferase (U/L) | 36 ± 8.5 |
| Albumin (mmol/L)1 | 3.8 ± 0.15 |
| Prothrombin times (seconds prolonged)1 | 1.48 ± 0.8 |
| Maximum size of tumor (cm)1 | 14.1 ± 2.6 |
| Pugh-Child’s grading, n (%) | |
| A | 559 (88.2%) |
| B | 75 (11.8%) |
| C | 0 |
| Alpha fetoprotein ≥ 400 ng/mL | 310 (58.9%)2 |
| HBsAg positive | 469 (74.0%) |
| Satellite nodules | 139 (26.5%) |
| Tumor thrombi in portal vein | 139 (21.9%) |
| Associated cirrhosis | 547 (86.3%) |
| Type of liver resection | n | % |
| Resection of right three lobes | 30 | 4.8 |
| Resection of right half | 44 | 6.9 |
| Resection of left three lobes | 39 | 6.2 |
| Resection of left half | 63 | 9.9 |
| Segmentectomy 4, 5, 6 | 33 | 5.2 |
| Segmentectomy 4, 5, 8 | 27 | 4.2 |
| Segmentectomy 5, 6, 7 | 87 | 13.7 |
| Segmentectomy 2, 3 | 128 | 20.2 |
| Non-anatomical resections | 183 | 28.9 |
| Total | 634 | 100 |
| Combined organ resection | ||
| Partial diaphragamectomy | 12 | 1.9 |
| Right adrenal gland resection | 9 | 1.4 |
| Distal gastrectomy | 5 | 0.8 |
| Transverse colectomy | 11 | 1.7 |
| Splenectomy | 31 | 4.9 |
| Splenectomy + devascularization | 41 | 6.5 |
| Portal vein thrombectomy | 139 | 21.9 |
| Total | 248 | 39.1 |
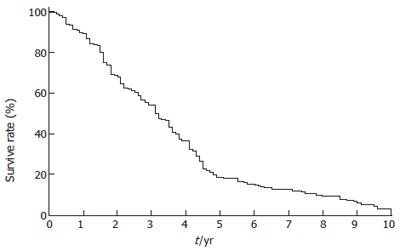
The operative time was 98 ± 41 min, inflow occlusion time was 10.5 ± 2.7 min. Blood loss was 440 ± 250 mL and transfusion requirement was 480 ± 350 mL. Postoperative complications were common, such as reactive right pleural effusion (n = 135 , 21.4%), significant ascites (n = 30, 4.7%),bleeding from stress ulceration (n = 11, 1.7%), postoperative hemorrhage on the surface of liver (n = 7, 1.3%), infected wound (n = 10, 1.6%), bile leakage (n = 7, 1.1%) and intraabdominal abscess (n = 6, 0.9%). Re-laparotomy to deal with the complications was required in 5 patients (0.8%). The 30-d mortality rate was 2.2%, 9 of 14 patients died of liver failure, 4 of 14 died of postoperative bleeding, and 1 died of septic complication. The 3-, 5- and 10-year survival rates were 35.1%, 18.2% and 3.5%, respectively (Figure 1).
Univariate analysis showed the following prognostic factors associated with significant improvement in survival: female sex, low serum alpha fetoprotein of less than 400 ng/mL, presence of tumor capsule, absence of extensive capsular infiltration by tumor, absence of tumor thrombus in main portal vein and satellite nodules (Table 3). On multivariate analysis, the significant prognostic factors for long-term survival were extensive capsular infiltration, tumor thrombus in portal vein, satellite nodules and intraoperative blood loss (Table 4).
| Variable | Patients(n) | Survival rates (%) | |||||
| 1 yr | 2 yr | 3 yr | 5 yr | ||||
| Patients | |||||||
| Sex | Male | 332 | 65.1 | 43.7 | 33.7 | 15.1 | P < 0.05 |
| Female | 24 | 83.3 | 70.8 | 45.8 | 37.5 | ||
| Age | < 50 yr | 206 | 64.5 | 42.7 | 32.0 | 15.1 | NS |
| ≥ 50 yr | 150 | 68.7 | 46.3 | 38.0 | 18.7 | ||
| 1AFP ng/mL | < 400 | 130 | 68.4 | 49.2 | 37.7 | 19.2 | P < 0.05 |
| ≥ 400 ng/mL | 154 | 62.3 | 32.5 | 26.0 | 9.1 | ||
| Tumor size | |||||||
| 10-15 cm | 281 | 71.5 | 46.6 | 39.5 | 17.4 | P < 0.05 | |
| > 15 cm | 75 | 46.7 | 41.3 | 16.0 | 0 | ||
| Capsule | |||||||
| Positive | 270 | 71.0 | 48.1 | 44.0 | 21.9 | P < 0.05 | |
| Negative | 86 | 48.8 | 37.2 | 4.7 | 0 | ||
| Extensive capsular infiltration | |||||||
| Positive | 224 | 67.4 | 40.6 | 39.3 | 12.1 | P < 0.05 | |
| Negative | 46 | 93.5 | 84.8 | 76.1 | 69.6 | ||
| Tumor thrombus in portal vein | |||||||
| Positive | 62 | 38.7 | 22.6 | 6.5 | 0 | P < 0.05 | |
| Negative | 294 | 72.1 | 50.3 | 40.5 | 20.1 | ||
| Satellite nodules | |||||||
| Positive | 87 | 36.8 | 23.0 | 12.6 | 0 | P < 0.01 | |
| Negative | 269 | 75.8 | 52.8 | 41.6 | 21.9 | ||
| Surgical resection margin | |||||||
| ≤ 10 mm | 251 | 64.1 | 40.2 | 34.3 | 13.9 | NS | |
| > 10 mm | 105 | 71.4 | 58.1 | 35.2 | 22.9 | ||
| Intraoperative blood loss | |||||||
| ≤ 800 mL | 260 | 69.6 | 43.8 | 33.5 | 15.4 | NS | |
| > 800 mL | 96 | 57.3 | 50.0 | 37.5 | 19.8 | ||
| Factor(variable) | Coefficient ofregression | Relativerisk | Standarderror | Walesstatistic | P |
| Capsule | |||||
| Positive | -1.5152 | 1.3521 | 0.5078 | 9.543 | < 0.05 |
| Negative | |||||
| Extensive capsular infiltration | |||||
| Positive | -2.5237 | 1.4152 | 0.4035 | 10.321 | < 0.05 |
| Negative | |||||
| Tumor thrombus in portal vein | |||||
| Positive | -3.1012 | 2.4240 | 0.7122 | 7.543 | < 0.05 |
| Negative | |||||
| Satellite nodules | |||||
| Positive | -2.5158 | 1.7852 | 0.8055 | 7.257 | < 0.05 |
| Negative | |||||
| Intraoperative blood loss | |||||
| ≤ 800 mL | -5.5257 | 1.5023 | 0.7211 | 15.15 | < 0.05 |
| > 800 mL | |||||
HCC usually presents at a very late stage in its natural history because of the lack of symptoms in the early stage. The large size and the position of the liver behind the costal cartilages preclude the tumor from being readily palpable during early stage. Furthermore, the large functional reserve of the liver delays clinical presentation with functional disturbances of the liver. Some patients present with large tumors in the liver in their first visits.
The tumor size is a significant risk factor for intrahepatic and extrahepatic spread[14-16]. Whether the tumor’s greatest diameter impacts on the disease-free interval and the overall survival remains unclear. Even when small HCCs are examined as a specific group, a better survival can still be observed with decreasing size of the tumor after resection[7,9]. The frequency of intrahepatic metastasis increases by almost a third between HCC smaller and larger than 5 cm and the rate of portal vein tumor thrombosis is almost doubled[15,16]. There is little information showing that patients with huge HCC over 10 cm in diameter without any extrahepatic metastases have long-term results after liver resection. Our study on 634 patients showed a 5-year survival of 18.2%. It is low when compared with reported series of patients with HCC unselected for the tumor size, the overall 5-year survival of these series ranges from 11% to 76% with a median around 30%, which might be due to the more advanced stage of cancer at the time of surgery.
One of the major concerns in operating on huge HCC is the operative mortality because of the extent of the liver resection, which is commonly involved in these huge tumors. The 30-d mortality of our patients was 2.2% which is low compared with that reported in the medical literature on HCC ranging from 0.5% to 21.5%, with a median of around 5% to 10%. Our low operative mortality is probably related to the surgeons’ experience and careful case selection.
Intraopertive blood loss can adversely affect the perioperative mortality of patients undergoing liver resection[18-20]. There is evidence that blood transfusion may be associated with an increased risk of recurrence in patients operated for a hepato-biliary malignancy, due to impairment of the patient’s immune response[21]. Our method of liver resection[11,12] can offer much to patients with huge HCC. As discussed previously, our technique not only cuts down the intraoperative bleeding, but also follows oncological principles. The other factor related to our good results is careful selection of patients. In cirrhotic liver resection, the onset of postoperative liver failure and mortality is related to the degree of preoperative liver failure[22] and the extent of non-tumor liver parenchyma removed[23]. An ideal candidate for liver resection in a patient with huge HCC is a patient with good liver function without ascites (Pugh-Child A).
Using univariate and multivariate analyses, we identified several prognostic factors, associated with a better long-term survival for patients with huge HCC after live resection. However, all these factors reflect the biologic behavior of the tumor, and cannot be used to exclude patients from liver resection with an intention for a cure. They can be used as a guide to make decisions in balancing the risks of the operation against the potential benefits in a patient who has increased risks of mortality because of his general condition or borderline liver functions. In cirrhotic liver resection, resecting too generous a margin can result in a higher chance of postoperative liver failure. However, a negative histological margin is the only factor that the surgeon can control the long-term survival results. Therefore the tumor should be resected aiming at a 1cm gross margin although our study showed resection margin of above or below 1 cm had no impact on long-term survival provided that the histological margin was clear.
In conclusion, in carefully selected patients, liver resection in patients with HCC of 10 cm or more in diameter is safe.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389-399. [PubMed] |
| 2. | Blumgart LH, Leach KG, Karran SJ. Observations on liver regeneration after right hepatic lobectomy. Gut. 1971;12:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Miyagawa S, Kawasaki S, Noike T, Nomura K, Kobayashi A, Shimada R, Imamura H. Liver regeneration after extended right hemihepatectomy in patients with hilar or diffuse bile duct carcinoma. Hepatogastroenterology. 1999;46:364-368. [PubMed] |
| 4. | Yamanaka N, Okamoto E, Toyosaka A, Mitunobu M, Fujihara S, Kato T, Fujimoto J, Oriyama T, Furukawa K, Kawamura E. Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis. Cancer. 1990;65:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Sugioka A, Tsuzuki T, Kanai T. Postresection prognosis of patients with hepatocellular carcinoma. Surgery. 1993;113:612-618. [PubMed] |
| 6. | Lehnert T, Otto G, Herfarth C. Therapeutic modalities and prognostic factors for primary and secondary liver tumors. World J Surg. 1995;19:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Tang ZY, Yu YQ, Zhou XD, Ma ZC, Yang R, Lu JZ, Lin ZY, Yang BH. Surgery of small hepatocellular carcinoma. Analysis of 144 cases. Cancer. 1989;64:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Kawano N, Ohmori Y, Inoue S, Nagao T, Morioka Y. Long-term prognosis of surgical patients with hepatocellular carcinoma. Cancer Chemother Pharmacol. 1989;23 Suppl:S129-S132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Chen XP, Wu ZD, Qiu FZ. Right hemihepatectomy of directly tie off afferent and efferent hepatic vessels of the bearing-bearing liver. Zhonghua Shiyong Waike Zazhi. 1999;19:678-679. |
| 12. | Chen XP, Wu ZD, Qiu FZ. Left hemiheptectomy of directly tie off afferent and efferent hepatic vessels of the bearing-bearing liver. Dig Surg. 1999;2:69-70. |
| 13. | Launois B. The intrahepatic Glissonian approach to liver resection. In : Blumgart LH, Fong Y (eds) Surgery of the Liver and Biliary Tract. London: WB Saunders 2000; 1698-1703. |
| 14. | The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 271] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Lau WY. Primary hepatocellular carcinoma. London: WB Saunders 2000; 1423-1450. |
| 18. | Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Makuuchi M, Takayama T, Gunvén P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Shimada M, Matsumata T, Akazawa K, Kamakura T, Itasaka H, Sugimachi K, Nose Y. Estimation of risk of major complications after hepatic resection. Am J Surg. 1994;167:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, Adson MA. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493-504; discussion 504-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 313] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Franco D, Capussotti L, Smadja C, Bouzari H, Meakins J, Kemeny F, Grange D, Dellepiane M. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology. 1990;98:733-738. [PubMed] |
| 23. | Stone HH, Long WD, Smith RB 3rd, Haynes CD. Physiologic considerations in major hepatic resections. Am J Surg. 1969;117:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 1.6] [Reference Citation Analysis (0)] |