Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4546
Revised: March 8, 2006
Accepted: March 27, 2006
Published online: July 28, 2006
AIM: To analyze the percentages of hepatocytes with increased nuclear DNA content, i.e., tetraploid (4n) and octoploid (8n) nuclei, and then compared mononuclear and binuclear hepatocyte populations.
METHODS: The percentages of mononuclear diploid (2n), 4n, and 8n hepatocytes and those of binuclear 2 × 2n, 2 × 4n, and 2 × 8n hepatocytes were determined with a method that can simultaneously measure hepatocyte nuclear DNA content and binuclearity in 62 patients with chronic hepatitis B or C. The percentage of 4n and 8n hepatocytes in the mononuclear hepatocyte population was compared with the percentage of 2 × 4n and 2 × 8n hepatocytes in the binuclear hepatocyte population.
RESULTS: The percentages of 4n and 8n hepatocytes in mononuclear hepatocytes and 2 × 4n and 2 × 8n hepatocytes in binuclear hepatocytes were similar, regardless of the activity or fibrosis grade of chronic hepatitis and regardless of the infecting virus.
CONCLUSION: The distribution of nuclear DNA content within mononuclear and binuclear hepatocyte populations was conserved during the course of chronic viral hepatitis.
- Citation: Toyoda H, Kumada T, Bregerie O, Brechot C, Desdouets C. Conserved balance of hepatocyte nuclear DNA content in mononuclear and binuclear hepatocyte populations during the course of chronic viral hepatitis. World J Gastroenterol 2006; 12(28): 4546-4548
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4546
Hepatocyte polyploidization and binucleation are two important features of liver growth and physiology. In adult humans, the number of polyploid liver cells is reportedly 20%-45%[1,2]. The importance of controlling hepatocyte polyploidization in both normal and pathological liver conditions has been reported. During postnatal growth, the liver parenchyma undergoes dramatic changes characterized by gradual polyploidization[3]. In adults, hepatocyte polyploidization is differentially regulated upon loss of liver mass and liver damage. Partial hepatectomy induces marked cell proliferation followed by an increase in hepatocyte ploidy[4]. In contrast, in different liver pathologies, such as hepatocellular carcinoma, growth shifts to a non-polyploidizing pattern and expansion of the diploid hepatocyte population is observed[5].
We previously studied changes in hepatocyte ploidy and binuclearity profiles in patients with chronic viral hepatitis and found that the percentage of diploid hepatocytes was significantly reduced in patients with high hepatitis activity and marked fibrosis and that the polyploid hepatocyte fraction was increased in these patients[6]. Thus, during chronic hepatitis, the changes in ploidization is similar to that observed after partial hepatectomy in adults. In this previous study, we validated a technique that allows simultaneous measurement of hepatocyte nuclear DNA content and hepatocyte binuclearity in the same liver sections. This is a technique that makes it possible to evaluate the DNA content of a binuclear hepatocyte.
In the present study, we used the same technique to analyze the nuclear DNA contents of mononuclear and binuclear hepatocytes, to compare the changes in the distribution of hepatocytes with different nuclear DNA content between mononuclear and binuclear hepatocyte populations during the course of human chronic hepatitis.
A total of 62 patients with chronic viral hepatitis (44 men and 18 women, mean age 39.6 ± 5.7 years) were studied. An ultrasonography-guided needle liver biopsy had been performed on all patients between March 1996 and March 2001. Twenty-seven patients were chronically infected with hepatitis B virus (HBV), whereas the other 35 patients were infected with hepatitis C virus (HCV). On the basis of histological findings, hepatitis activity as determined with the METAVIR scoring system[7] was A0 in 15 patients, A1 in 13 patients, A2 in 13 patients, and A3 in 21 patients, and the degree of fibrosis was F0 in 7 patients, F1 in 21 patients, F2 in 11 patients, and F3 in 23 patients.
Analyses of hepatocyte nuclear DNA content and hepatocyte binuclearity were performed as described previously[6,8,9]. Three-micrometer-thick, paraffin-embedded liver tissue sections were incubated for 1 h with anti-cytokeratin antibody (1:50, KL1, Immunotech, S.A., Marseille, France) as the primary antibody for immunostaining of hepatocyte membrane, followed by a 30-min incubation with biotinylated swine anti-goat, mouse, rabbit immunoglobulin solution (1:200, Dako) and a 30-min incubation with FITC-conjugated streptavidin (1:200, Dako). Sections were then stained for 20 min with Hoechst 33342 (1 mg/L) to stain DNA. Tissue sections were then examined under a Zeiss inverted microscope (Axiovert 35, Carl Zeiss, Gottingen, Germany) equipped for epi-illumination. Images were captured with a cooled, charged coupled device (CCD) camera (KAF 1400-G2, class 2, Photometrics, Tucson, AZ) on 4056 grey levels. Automatic quantitative image analysis was performed in 12 bits with the IPLab Spectrum version 3.1 software. Hepatocytes were classified as mono- or binuclear hepatocytes on the basis of comparisons of fluorescent and membrane labelling images. Other liver cell types, which were defined by morphologic characteristics, were eliminated. Integrated fluorescence intensity was stored in computer files for analysis. A histogram of the fluorescence intensity per section was drawn, and the distribution of each hepatocyte population (2n [diploid] DNA content as the first peak on the histogram, 4n [tetraploid] DNA content as the second peak on the histogram, and 8n [octoploid] DNA content as the third peak on the histogram) was calculated. To develop a histogram of the fluorescence intensity of binuclear hepatocytes, we used the fluorescence intensity of the nucleus with the clearest edge when only one of the two nuclei of a binuclear hepatocyte had a clear edge on the image analyzed. When two nuclei had clear edges, we calculated the average of fluorescence intensity of the two nuclei and used this value as fluorescence intensity of the hepatocyte. A minimum of 300 hepatocytes on eight to 12 separate fields was studied. To ensure that the same cells were not counted twice, slides were read by two observers in a systematic manner of moving from right to left along the slide and then along successive descending lines. All analyses were performed blind to any sample-specific data.
Correlations between values were analyzed by Spearman test, and a P < 0.05 was accepted as statistically significant. The study was approved by the Institutional Review Board of Hopital Necker-Enfants Malades (Paris, France) and was carried out in accordance with the Helsinki declaration.
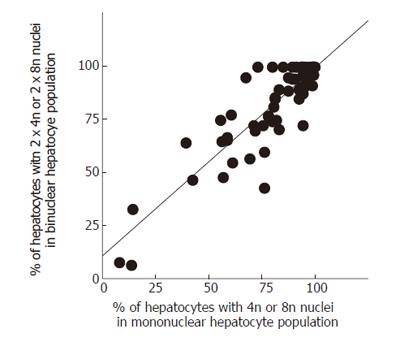
We analyzed the percentages of binuclear hepatocyes with diploid (2n) nuclei and those with tetraploid (4n) or octoploid (8n) nuclei (namely binuclear 2 × 2n and 2 × 4n + 2 × 8n) and compared them with the percentages of mononuclear hepatocyte with 2n nucleus and those with 4n or 8n nucleus (namely mononuclear 2n and 4n + 8n) in samples from patients with chronic viral hepatitis. We found a significant correlation between the percentages of mononuclear 4n and 8n hepatocytes and binuclear 2 × 4n and 2 × 8n hepatocytes (P < 0.0001; correlation coefficient, 0.826 [0.726-0.892]); the percentages of nuclei of 4n or 8n DNA content was maintained in the mononuclear hepatocyte population and in the binuclear hepatocyte population (Figure 1). This correlation was maintained when we focused specifically on patients with HBV or HCV. The correlation was also maintained regardless of activity of hepatitis, grade of liver fibrosis, age, or sex (data not shown). In contrast, there was no correlation between the percentage of binuclear 2 × 4n and 2 × 8n hepatocyes and the percentage of binuclear hepatocytes in the total hepatocyte population (P = 0.1276; correlation coefficient, 0.196 [-0.057-0.425]; Figure 2). The percentage of binuclear hepatocytes with increased nuclear DNA content (2 × 4n and 2 × 8n hepatocytes), therefore, was not associated with the percentage of binuclear hepatocytes in the total hepatocyte population.
Flow cytometry can measure nuclear DNA content but cannot distinguish between nuclei of mononuclear hepatocytes and those of binuclear hepatocytes. In addition, morphologic observation of hematoxylin and eosin-stained sections can distinguish mononuclear and binuclear hepatocytes but cannot quantify nuclear DNA content. To our knowledge, the present study is the first to compare the distributions of nuclear DNA content between mononuclear and binuclear hepatocyte populations with a novel method that allowed evaluation of the distribution of nuclear DNA content (2 × 2n, 2 × 4n, and 2 × 8n) exclusively in binuclear hepatocytes.
With respect to the hypothesis that mononuclear hepatocytes are stimulated to form binuclear hepatocytes, our findings suggest that both diploid and polyploid mononuclear hepatocytes are similarly stimulated to form binuclear hepatocytes, suggesting the possibility that the formation of binuclear hepatocytes is regulated by a single factor for both diploid and polyploid hepatocytes. Alternatively, it is possible that both diploid and polyploid hepatocytes are sensitive to a single stimulus for binuclear hepatocyte formation. This appears to be independent of viral infection and is maintained throughout the course of chronic hepatitis.
In a previous report[6], we showed that the decrease in the percentage of diploid hepatocytes was correlated with the progression of chronic hepatitis. In addition, we showed there was a higher percentage of binuclear hepatocytes in patients with HBV infection than those with HCV infection. Our present results indicate that the percentage of diploid hepatocytes decreases in both mononuclear hepatocyte and binuclear hepatocyte populations, in a similar way according to the progression of chronic hepatitis. Also, the present data indicate that binuclear hepatocytes increase in patients infected with HBV, conserving the distribution of different nuclear DNA content (2 × 2n, 2 × 4n, and 2 × 8n hepatocytes).
The mechanisms that underlie hepatocyte polyploidization are still largely unknown. The importance of binuclear hepatyocyte formation as a step towards hepatocyte polyploidization has been reported. We previously provided direct evidence for the pivotal role of binuclear hepatocytes in the formation of 4n mononuclear hepatocytes, indicating a close association between binuclear hepatocyte formation and polyploid mononuclear hepatocyte formation in vitro[9]. Our present study indicated that formation of polyploid mononuclear hepatocytes is associated with formation of polyploid binuclear hepatocyte in human chronic viral hepatitis. Further studies are needed to clarify the mechanisms that control hepatocyte polyploidization and binucleation during human chronic viral hepatitis. In addition, the influence of steatosis, which is often observed in case of chronic hepatitis C and can reduce replication in hepatocytes, on hepatocyte polyploidization and binucleation should be elucidated in the future.
S- Editor Pan BR L- Editor Lutze M E- Editor Bi L
| 1. | Kudryavtsev BN, Kudryavtseva MV, Sakuta GA, Stein GI. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Melchiorri C, Bolondi L, Chieco P, Pagnoni M, Gramantieri L, Barbara L. Diagnostic and prognostic value of DNA ploidy and cell nuclearity in ultrasound-guided liver biopsies. Cancer. 1994;74:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Gupta S. Hepatic polyploidy and liver growth control. Semin Cancer Biol. 2000;10:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Gerlyng P, Abyholm A, Grotmol T, Erikstein B, Huitfeldt HS, Stokke T, Seglen PO. Binucleation and polyploidization patterns in developmental and regenerative rat liver growth. Cell Prolif. 1993;26:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Saeter G, Lee CZ, Schwarze PE, Ous S, Chen DS, Sung JL, Seglen PO. Changes in ploidy distributions in human liver carcinogenesis. J Natl Cancer Inst. 1988;80:1480-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Toyoda H, Bregerie O, Vallet A, Nalpas B, Pivert G, Brechot C, Desdouets C. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut. 2005;54:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 8. | Lamas E, Chassoux D, Decaux JF, Brechot C, Debey P. Quantitative fluorescence imaging approach for the study of polyploidization in hepatocytes. J Histochem Cytochem. 2003;51:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Guidotti JE, Brégerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278:19095-19101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |