Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4541
Revised: January 10, 2006
Accepted: January 24, 2006
Published online: July 28, 2006
AIM: To evaluate the levels of serum carnitine in patients with cancer in digestive organs and to compare them with other cancers in order to provide new insights into the mechanisms of cachexia.
METHODS: Fifty-five cachectic patients with or without gastrointestinal cancer were enrolled in the present study. They underwent routine laboratory investigations, including examination of the levels of various forms of carnitine present in serum (i.e., long-chain acylcarnitine, short-chain acylcarnitine, free carnitine, and total carnitine). These values were compared with those found in 60 cancer patients in good nutritional status as well as with those of 30 healthy control subjects.
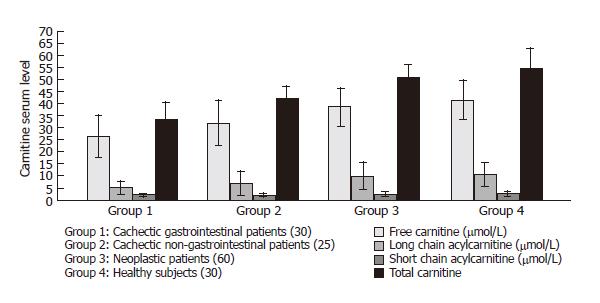
RESULTS: When the cachectic patients with gastro-intestinal cancer were compared with the cachectic patients without gastrointestinal cancer, the difference was -6.8 μmol/L in free carnitine (P < 0.005), 0.04 μmol/L in long chain acylcarnitine (P < 0.05), 8.7 μmol/L in total carnitine (P < 0.001). In the cachectic patients with or without gastrointestinal cancer, the difference was 12.2 μmol/L in free carnitine (P < 0.001), 4.60 μmol/L in short chain acylcarnitine (P < 0.001), and 0.60 μmol /L in long-chain acylcarnitine (P < 0.005) and 17.4 μmol/L in total carnitine (P < 0.001). In the cachectic patients with gastrointestinal cancer and the healthy control subjects, the difference was 15.5 μmol/L in free carnitine (P < 0.001), 5.2 μmol /L in short-chain acylcarnitine (P < 0.001), 1.0 μmol/L in long chain acylcarnitine (P < 0.001), and 21.8 μmol/L in total carnitine (P < 0.001).
CONCLUSION: Low serum levels of carnitine in terminal neoplastic patients are decreased greatly due to the decreased dietary intake and impaired endogenous synthesis of this substance. These low serum carnitine levels also contribute to the progression of cachexia in cancer patients.
- Citation: Malaguarnera M, Risino C, Gargante MP, Oreste G, Barone G, Tomasello AV, Costanzo M, Cannizzaro MA. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J Gastroenterol 2006; 12(28): 4541-4545
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4541
Cancer cachexia is a condition characterized by anorexia, chronic nausea, early satiety, muscle wasting, involuntary weight loss, lipolysis, weakness, fatigue, impaired mental and physical performance, decreased capacity of wound healing, impaired immunological function, and compromized quality of life. Cancer cachexia is associated with poor survival and decreased tolerance to both radiotherapy and chemotherapy[1].
The prevalence of cachexia is about 60% in terminal cancer patients[2]. Anorexia and nausea exacerbate the detrimental effects of tumor-related changes in protein metabolism on nutritional status, leading to increased morbidity and mortality[3].
The mechanisms responsible for cachexia in cancer patients are poorly understood. One of the factors contributing to weight loss is the reduced food intake, which may be caused by a decrease in appetite or a tumor treatment[4], mechanical obstruction of the gastrointestinal (GI) tract or intestinal malabsorption.
A decrease in food intake, combined with a decrease in physical exercise, leads to a decline in muscle mass and power.
Several studies have suggested that metabolic control of food intake also exists, in which the biochemical partitioning between fatty acid oxidation and synthesis is a vital signal indicating catabolic or anabolic energy status[5]. Changes in energy metabolism influence energy intake via energy signals.
Energy signals are independent from body mass, but they inform the brain on the metabolic switch occurring subcellularly between fatty acid oxidation and synthesis[6]. The combination of reduced energy intake and increased energy expenditure in cancer patients results in substantial weight loss. It has been shown that weight loss in cancer patients comprises both muscle mass and fat mass.
When there is a cancer, several metabolic changes are present in the whole body. It could be possible that one of these factors is represented by a decrease of endogenous synthesis of carnitine or by a reduction of exogenous assumption of carnitine.
Carnitine is a naturally occurring substance required for energy metabolism. In omnivores approximately 75% of carnitine sources come from diet and 25% from endogenous synthesis[7]. Under normal conditions, omnivores absorb about 70%-80% of dietary carnitine[8].
Human skeletal muscle, heart, liver, kidney and brain are capable of biosynthesizing carnitine from motioning and lysine to its immediate precursor gamma butyrobetaine[9].
Carnitine is essential for the transport of long chain fatty acids across the mitochondrial membrane for fatty degradation and energy production and has the ability to shuttle short chain fatty acids from inside the mitochondria to the cytosol.
In a previous study[10], we observed low serum levels of carnitine in patients with tumoral cachexia, which may be due to a decreased availability of carnitine in the diet or to the altered endogenous biosynthesis.
The aim of the present study was to evaluate the levels of serum carnitine in patients with cancer in digestive organs and to compare them with other cancers in order to provide new insights into the mechanisms of cachexia.
Fifty-five patients eligible for this study had advanced malignancies localized in various parts of the body. The patients were in the terminal phase of their disease and only palliation was requested. All of them showed a weight loss above 5% in the 6 mo prior to enrollment in the study. Thirty cachectic patients with gastrointestinal cancer (16 males and 14 females, mean age 47.4 ± 7.6 years) had the following diagnoses: cancer of the stomach in 6, cancer of the small bowel in 6, cancer of the colon in 10, cancer of the rectum in 8. Twenty cachectic patients without gastrointestinal cancer (13 males and 12 females, mean age 49.1 ± 6.4 years) had the following diagnoses: lung cancer in 5, renal cell carcinoma in 5, malignant melanoma in 5, bladder cancer in 5, prostate cancer in 2, and breast cancer in 3. Forty of them underwent surgical intervention, while 31 received one or more chemotherapeutic treatments and 6 received radiotherapy. We also enrolled two groups of control subjects.
Sixty cancer patients were in good nutritional status (25 males and 35 females, mean age 48.6 ± 8.4 years). Their diagnoses were as follows: colorectal carcinoma in 10, lung cancer in 15, breast cancer in 10, gastric cancer in 3, bladder cancer in 7, renal cell carcinoma in 4, prostate cancer in 10, and testicular cancer in 1.
A second control group consisted of 30 healthy individuals aged 51.3 ± 5.2 years. The patient group (n = 4) was examined in the morning between 8:00 a.m. and 10:00 a.m. after an overnight fast. Then, venous blood samples were taken and stored in tubes containing ethylenediamine tetra acetic acid (EDTA) or heparin (Table 1).
| Parameter | Cachectic patients with gastrointestinal cancer (30) | Cachectic patients without gastrointestinal cancer (25) | Non cachectic patients with cancer(60) | Healthy subjects(30) |
| Sex M/F | 16/14 | 13/12 | 25/35 | 15/15 |
| Mean age (yr) | 47.4 ± 7.6 | 49.1 ± 6.4 | 48.6 ± 8.4 | 51.3 ± 5.2 |
| Heigth (cm) | 158.9 ± 6.8 | 160.6 ± 7.1 | 159.4 ± 7.8 | 160.2 ± 6.4 |
| Weigth (kg) | 46.8 ± 7.6 | 51.4 ± 6.9 | 60.2 ± 7.4 | 63.8 ± 11.3 |
| SAP (mmHg) | 151.8 ± 12.4 | 156 ± 13.2 | 152.4 ± 14.7 | 150.3 ± 16.8 |
| DAP (mmHg) | 84.2 ± 8.7 | 86.4 ± 7.1 | 80.1 ± 9.7 | 80.2 ± 10.6 |
| Heat Rate bpm | 94.2 ± 9.8 | 86.2 ± 11.1 | 84.2 ± 7.1 | 81.2 ± 10.2 |
Serum or plasma was obtained by centrifugation. Serum was analyzed immediately, while plasma and urine were stored at -20°C before analysis. Serum carnitine levels were determined using the Cederblad and Lindstedt method modified by Brass and Hoppel[7]. Plasma was treated with perchloric acid (final concentration 3% vol:vol) and centrifuged for 2 min at 10 000 r/min. Long-chain acylcarnitine was extracted from the pellet after alkaline hydrolysis, while free and short-chain acylcarnitines were extracted from the supernatant. The sum of short-chain acylcarnitine, free carnitine, and long-chain acylcarnitine was considered the serum of total carnitine level.
Creatinine concentrations were determined using a kinetic colorimetric reaction in the same samples used to measure the carnitine concentrations. All laboratory tests were performed using standard laboratory procedures.
All subjects followed a daily diet consisting of 1800 kcal/d with a content of total cholesterol < 300 mg/d, 50% carbohydrates, 20% proteins, and 30% fats (of which 10% were saturated fatty acids, 10% unsaturated fatty acids, and 10% polyunsaturated fatty acids).
The results are presented as mean ± SD. The following two-tailed tests at P < 0.05 were used in the study: the Mann-Whitney U-test in the case of two independent samples and the Spearman’s rank correlation coefficient test for univariate relationships between variables. In order to evaluate the independent effects of covariates on carnitine concentration, a stepwise multiple linear regression analysis was performed.
Data were analyzed using the statistical package SPSS for Windows 7.5 (SPSS Inc., Chicago, IL, USA).
The four groups of subjects were comparable in age, height, systolic and diastolic pressure. The difference in weight was 4.50 kg (P < 0.05) between cachectic patients with gastrointestinal cancer and cachectic patients without gastrointestinal cancer, 13.40 kg (P < 0.001) between cachectic patients with gastrointestinal cancer and neoplastic non-cachectic patients, and 17.0 kg between cachectic patients with gastrointestinal cancer and healthy subjects (P < 001). The difference in weight was 8.80 kg (P < 0.001) between cachectic patients without gastrointestinal cancer and non-cachectic patients, and 12.40 kg (P < 0.001) between cachectic patients without gastrointestinal cancer and healthy subjects.
When the cachectic patients with gastro-intestinal cancer were compared to the cachectic patients without gastrointestinal cancer, the difference was 12.5 mg/dL in triglycerides (P < 0.001), 26.7 IU/L in ALP (P < 0.001) and 802.6 ng/mL in CEA (P < 0.001).
The difference was 6.8 μmol/L in free carnitine (P < 0.005), 0.04 μmol/L in long chain acylcarnitine (P < 0.05), 8.7 μmol/L in total carnitine (P < 0.001).
When the cachectic patients with gastrointestinal cancer were compared to non cachectic patients with cancer, the difference was 5.8 mg/dL in bun (P < 0.001), 7.00 mg/dL in glucose (P < 0.001), 34.1 mg/dL in total cholesterol (P < 0.001), 31.3 mg/dL in triglycerides (P < 0.001), 8.60 IU/L in AST (P < 0.001), 45.8 IU/L in ALP (P < 0.001) and 815.6 ng/mL in CEA (P < 0.001). The difference was 12.2 μmol/L in free carnitine (P < 0.001), 4.60 μmol/L in short-chain acylcarnitine (P < 0.001), and 0.60 μmol/L in long-chain acylcarnitine (P < 0.005) and 17.4 μmol/L in total carnitine (P < 0.001).
When the cachectic patients with gastrointestinal cancer were compared to the healthy control subjects, the difference was 27.60 mg/dL in total cholesterol (P < 0.001), 25.8 mg/ dL in triglycerides (P < 0.001), 13.0 IU/L in ALT (P < 0.001), 15.60 IU/L in AST (P <0.001), and 76.8 IU/L in ALP (P < 0.001) and 845.1 ng/mL in CEA (P < 0.001). The difference was 15.5 μmol/L in free carnitine (P < 0.001), 5.2 μmol/L in short-chain acylcarnitine (P < 0.001), 1.0 μmol/L in long-chain acylcarnitine (P < 0.001), and 21.8 μmol/L in total carnitine (P < 0.001).
When the cachectic patients without gastrointestinal cancer were compared to the non cachectic patients with cancer, the difference was 3.2 mg/dL in bun (P < 0.01), 7.5 mg/dL in glucose (P < 0.001), 27.3 mg/dL in total cholesterol (P < 0.001), 18.8 mg in triglycerides (P < 0.001), 5.9 IU/L in AST (P < 0.01), 19.10 in ALP (P < 0.001), 5.40 μmol/L in free carnitine (P < 0.01), 3.10 μmol/L in short-chain acylcarnitine (P < 0.005), 8.7 μmol/L in total carnitine (P < 0.001).
When the cachectic patients without gastrointestinal cancer were compared to the healthy controls, the difference was 5.40 mg/dL in Bun (P < 0.005), 20.8 mg/dL in total cholesterol (P < 0.001), 13.3 mg/dL in triglycerides (P < 0.001), 8.7 IU/L in ALT (P < 0.001), 12.9 IU/L in AST (P < 0.001), 50.1 IU/L in ALP (P < 0.001), 8.7 μmol/L in free carnitine (P < 0.001), 3.7 μmol/L in short-chain acylcarnitine (P < 0.01), 0.6 μmol/L in long-chain acylcarnitine L (P < 0.005), 13.1 μmol/L in total carnitine (P < 0.001).
When non-cachectic cancer patients were compared to the healthy controls, the difference was 8.6 mg/dL in BUN (P < 0.001), 4.2 mg/dL in glucose (P < 0.05), 6.5 mg/dL in total cholesterol (P < 0.005), 5.5 mg in triglycerides (P < 0.01), 9.4 IU/L in ALT (P < 0.001), 7.00 IU/L in AST (P < 0.001), and 31.01 IU/L in ALP (P < 0.001).
In comparison of serum plasma carnitine in controls and neoplastic patients, there were no significant differences (Table 2).
| Parameter | Group 1(30 pts) | Group 2(25 pts) | Group 3 (60 pts) | Group 4(30 pts) |
| Bun (mg/dL) | 35.6 ± 5.4 | 38.2 ± 5.1 | 41.4 ± 4.7 | 32.8 ± 7.9 |
| Glucose (mg/dL) | 68.4 ± 9.1 | 67.9 ± 8.7 | 75.4 ± 9.1 | 71.2 ± 9.4 |
| Creatinine (mg/dL) | 0.87 ± 0.37 | 0.91 ± 0.25 | 1.06 ± 0.20 | 0.97 ± 0.21 |
| Total Cholesterol (mg/dL) | 160.1 ± 13.9 | 166.9 ± 14.2 | 194.2 ± 8.7 | 187.7 ± 10.8 |
| Triglycerides (mg/dL) | 144.3 ± 13.2 | 156.8 ± 10.9 | 175.6 ± 9.6 | 170.1 ± 9.8 |
| ALT (IU/L) | 49.7 ± 9.8 | 45.4 ± 7.9 | 46.1 ± 7.8 | 36.7 ± 4.4 |
| AST (IU/L) | 51.4 ± 8.9 | 48.7 ± 7.4 | 42.8 ± 9.6 | 35.8 ± 6.7 |
| ALP (IU/L) | 241.8 ± 22.6 | 215.1 ± 20.7 | 196 ± 16.4 | 165 ± 18.2 |
| CEA (ng/mL) | 847.2 ± 196.4 | 44.6 ± 13.1 | 31.6 ± 15.8 | 2.1 ± 1.4 |
The correlation studies in patients with gastrointestinal cancer showed a strong correlation between the following parameters: total carnitine (r = 0.75), short-chain acyl-carnitine (r = 0.70), long-chain acyl-carnitine (r = 0.78), free carnitine (R = 0.70), weight loss and total carnitine (R = 0.81), short chain acylcarnitine (r = 0.78), long chain acylcarnitine (r = 0.71), free carnitine (r = 0.74) and CEA.
The correlation studies in patients also showed a correlation between total carnitine (r = 0.61), short chain acylcarnitine (r = 0.65), and long chain acylcarnitine (r = 0.64) in free carnitine (r = 0.61) and weight loss.
No correlation was found between total carnitine or the fractions of carnitine and other biohumoral and demographic characteristics (Figure 1).
The current study demonstrated that patients with gastrointestinal cancer cachexia showed a significant decrease in fractions and total carnitine serum levels in comparison with controls.
Malignant lesions are generally associated with weight loss, due to delay in the establishment of a diagnosis. Gastrointestinal cancer is characterized by malabsorption with excess fecal loss of ions and water, in addition to nutrient. Cachexia is associated with inflammatory or neoplastic condition that evokes an acute-phase response with an increase of cytokine production (such tumor necrosis factor, interleukin 1, 6 and interferon), and feeding does not reverse the macronutrient changes.
Patients with gastrointestinal cancer may also experience anorexia secondary to food adversion.
Gastrointestinal cancer presents with symptoms and signs such as loss of appetite, abdominal discomfort, decreased gastrointestinal transit with obstructive symptoms, weight loss, weakness, nausea and vomiting.
Diversion to food can be attributed to the location of the tumor, its rate of growth and size[11].
Patients with growing tumors and poor intake due to bowel obstruction or severe dysphagia present with progressive nutritional deprivation as the main mechanism of cachexia.
The low plasma carnitine concentration in these patients is probably a consequence of decreased intake of carnitine and the carnitine precursors L-lysine and L-methionine.
Carnitine is critical for normal skeletal and heart muscle bioenergetics.
The decrease of carnitine and its derivates in patients with cachexia can explain the sarcopenia.
In fact, skeletal muscle is the main reservoir in the body and possesses a carnitine concentration at least 50 to 200 times higher than in blood, where the average concentration is about 50 μmol/L[12].
Administration of exogenous L- carnitine might improve the nutritional status in patients without gastrointestinal cancer and enhance muscle mass exerting a favorable effect on chronic fatigue syndrome in cancer patients.
The values of serum carnitine determination are questionable in respect to consequences and interpretation. This is due to the fact that serum carnitine represents approximately 3% of total body carnitine.
Rates of changes beyond the limits of 2%-3% in 1 mo can be considered abnormal. However, rates of changes within these limits may also be abnormal if divergent changes are seen in different body compartments (for example, depletion of skeletal muscle plus fluid overlood caused by cardiac, hepatic, or renal disease; hypoalbuminemia or intravenous hydration)[13,14] .
Carnitine is required for long-chain fatty acid oxidation and assists in removing accumulated acyl groups from the mitochondria and plays an important role in detoxification[15]. The reduced levels of carnitine seem to be related with both malabsorption and impaired carnitine production. A decrease in serum carnitine explains not only the sarcopenia but also indicates a bioenergetic deficit with the physical and mental fatigue detectable in patients[16].
An increase in resting energy expenditure may contribute to weight loss in cancer patients. L-carnitine has been shown to have physiologic effects on metabolism in cachexia models, presumably because of its ability to increase fatty oxidation[17].
The decrease in total serum carnitine is associated with a decrease in acylcarnitine, a fundamental substance in brain metabolism. Deficiency in acyl carnitine can induce behavioral and cognitive changes (anxiety, depression and malignancy or treatment-related anorexia)[18,19].
The direct relationship between total serum carnitine and short-chain and long-chain acylcarnitine levels in patients with gastrointestinal cancer cachexia and CEA suggests that the observed decrease in serum carnitine levels is directly influenced by the activity of gastrointestinal cancer.
In fact, malnutrition triggers a vicious circle in which neoplastic patients produce additional amounts of cytokines. The decrease in total and fractions of carnitine in patients without gastrointestinal cancer cachexia in comparison with cancer patients in good nutritional status and healthy control subjects suggests the alteration of endogenous biosynthesis of carnitine due to elevated cytokine production associated with cachexia. In fact, cachexia has a multifactorial pathogenesis and involves several neuronal systems that modulate production and transport of cell energy, such as hormones, neuropeptides, cytokines and neurotransmitters (serotonin and dopamine)[20].
Since therapeutic options currently available for the treatment of these patients are not successful,administration of carnitine may allow us to correct the unregulated immune response and improve the total energy expenditure[21]. The total energy expenditure involves in fact resting energy expenditure (approximately 70%), voluntary energy expenditure (25%), and energy expenditure in digestion (5%).
Therefore, there is no evidence that supports the use of nutritional or pharmacological intervention to improve the likelihood of either survival or improved anti-neoplastic interventions[22,23]. L-carnitine and L-acetylcarnitine may be effective at limiting the demands placed on cachectic patients by acute stresses, such as sudden increases in physical activity, immunological challenge or acute and chronic malnutrition.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | De Wyes WD, Herbst SH. Oral feeding in the nutritional management of the cancer patient. Cancer Res. 1977;37:2429-2431. [PubMed] |
| 2. | Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, Martini C, Monti M, Arnoldi E, Piva L, Ravaioli A. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:240-247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 279] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Bruera E, Sweeney C. Cachexia and asthenia in cancer patients. Lancet Oncol. 2000;1:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Pezner R, Archambeau JO. Critical evaluation of the role of nutritional support for radiation therapy patients. Cancer. 1985;55:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Kahler A, Zimmermann M, Langhans W. Suppression of hepatic fatty acid oxidation and food intake in men. Nutrition. 1999;15:819-828. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 722] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 7. | Rebouche CJ. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism. 1991;40:1305-1310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J Nutr. 1991;121:539-546. [PubMed] |
| 9. | Rebouche CJ, Engel AG. Tissue distribution of carnitine biosynthetic enzymes in man. Biochim Biophys Acta. 1980;630:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Vinci E, Rampello E, Zanoli L, Oreste G, Pistone G, Malaguarnera M. Serum carnitine levels in patients with tumoral cachexia. Eur J Intern Med. 2005;16:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg. 2000;66:46-51. [PubMed] |
| 12. | Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta. 2001;1546:21-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 265] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Roubenoff R, Heymsfield SB, Kehayias JJ, Cannon JG, Rosenberg IH. Standardization of nomenclature of body composition in weight loss. Am J Clin Nutr. 1997;66:192-196. [PubMed] |
| 14. | Leng SX, Erim E, McShine R, Bloom PA, Kotler DP. Influence of medical illness on body composition and quality of life in geriatric outpatients: a pilot study. J Am Geriatr Soc. 2000;48:1737-1738. [PubMed] |
| 15. | Karlic H, Lohninger S, Koeck T, Lohninger A. Dietary l-carnitine stimulates carnitine acyltransferases in the liver of aged rats. J Histochem Cytochem. 2002;50:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Pistone G, Marino A, Leotta C, Dell’Arte S, Finocchiaro G, Malaguarnera M. Levocarnitine administration in elderly subjects with rapid muscle fatigue: effect on body composition, lipid profile and fatigue. Drugs Aging. 2003;20:761-767. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Winter BK, Fiskum G, Gallo LL. Effects of L-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br J Cancer. 1995;72:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Neri S, Pistone G, Saraceno B, Pennisi G, Luca S, Malaguarnera M. L-carnitine decreases severity and type of fatigue induced by interferon-alpha in the treatment of patients with hepatitis C. Neuropsychobiology. 2003;47:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Malaguarnera M, Di Mauro A, Gargante PM, Rampello L. L-carnitine reduces severity of physical and mental fatigue and improves daily activities in the elderly. South Med J. 2006;99:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Laviano A, Rossi-Fanelli F. Pathogenesis of cancer anorexia: still doubts after all these years. Nutrition. 2003;19:67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Malaguarnera M, Maugeri D, Saraceno B, Romano M, Neri S, Rapisarda R, Pistone G. Effects of carnitine on biochemical responses in patients with chronic hepatitis C treated with interferon-α. Clin Drug Invest. 2002;22:443-448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Ripamonti C, Gemlo BT, Bozzetti F, De Conno F. Role of enteral nutrition in advanced cancer patients: indications and contraindications of the different techniques employed. Tumori. 1996;82:302-308. [PubMed] |
| 23. | Argiles JM, Meijsing SH, Pallares-Trujillo J, Guirao X, Lopez-Soriano FJ. Cancer cachexia: a therapeutic approach. Med Res Rev. 2001;21:83-101. [DOI] [Full Text] |