TO THE EDITOR
Sir, I read with great interest the recently published article in the World Journal of Gastroenterology by Jin and co-workers[1] on the cloning and characterization of porcine aquaporin 1 water channel from the pig liver and studies on its expression in the porcine gastrointestinal system. The authors should be congratulated for making this important and valuable contribution to the field of aquaporin biology and porcine gastrointestinal physiology. However, there are a number of unresolved issues and controversies concerning the expression of aquaporins (especially aquaporin 1) in the gastrointestinal system that are worthy of additional comment and discussion by Jin and co-workers.
It is now well established that aquaporin (AQP) water channels are a family of membrane proteins that facilitate water movement across cell membranes in plants and animals. Thus far, at least 13 human aquaporins (AQP0-AQP12) have been identified[2]. These proteins are selectively permeated by water (aquaporins)[3] or water plus glycerol (aquaglyceroporins)[4]. Aquaporins are strategically located at membrane sites in a variety of epithelial cells, most of which have well-defined transport functions; some are involved in fluid absorption, others in fluid secretion[5]. Aquaporin 1 (AQP1) was the first member of the AQP family to be identified, initially as a molecular component of the Colton blood group antigens in erythrocytes and subsequently cloned from kidney complementary DNA libraries and shown to possess water transporting activity[6,7]. Although AQP1 has been found to be important in osmotic water movement across cell membranes of many epithelial and endothelial barriers, its expression in the gastrointestinal system is exclusively limited to microvascular endothelia (Figure 1) and other aquaporin isoforms are known to be expressed in the epithelial cell lining. These aquaporins include AQP3[8-10], AQP4[11-13], AQP5[14,15], AQP8[16,17], AQP9[18] and AQP10[19-22]. It is therefore clearly established that multiple aquaporin isoforms are present along the gastrointestinal system; some of these are classical aquaporins (i.e., AQP1, AQP4, AQP5, AQP8) and are probably mainly involved in water homeostasis[23], whereas others are aquaglyceroporins (i.e., AQP3, AQP7, AQP9, AQP10) and are potentially involved in the facilitated movement of small uncharged organic molecules, such as glycerol[4]as well as water. In the fluid-transporting epithelia of the kidney nephron, AQP1 is permeated by water, driven by osmotic gradients and AQP1 is abundant in the apical and basolateral membranes of renal proximal tubules and descending thin limbs and plays a key role in setting up and maintaining the counter-current multiplication system[23,24]. Although AQP1 is known to be present in a number of extrarenal tissues, such as the ciliary body of the eye and the choroid plexus in the brain[6,23,25], it has not been reported in normal epithelial cells lining the gastrointestinal system[23]. The presence of AQP1 in gastrointestinal epithelial cells has only been reported in tumors of the colon, where it has been reported to contribute to tumor angiogenesis and the formation of high interstitial fluid pressures and high vascular permeability of tumor microvessels[26].
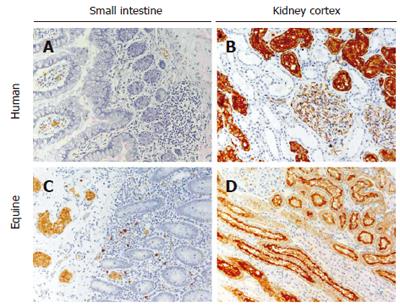
Figure 1 AQP1 expression in human and equine small intestine and kidney.
In the small intestine of both species, AQP1 is present exclusively in microvessel endothelia and in erythrocytes (panels A and C). AQP1 is not expressed in epithelial cells or any other structures in the small intestine (panels A and C). In the kidney of both species specific AQP1 expression is abundant in the renal cortex, specifically in the apical (brush border) and basolateral membranes of proximal tubules and in microvessel endothelia (panels B and D). Some AQP1 expression is also seen within the glomerulus in podocytes (panels B and D).
Jin et al[1] conclude that porcine AQP1 (pAQP1) is the first porcine aquaporin to be identified by means of molecular biology techniques. This is a valid and correct statement. However, the functional data they provide is from transfected CHO cells and red blood cells. The functional data presented by Jin et al[1] may not be used to support the statement that pAQP1 plays a key role in fluid transport in epithelial and endothelial structures of the pig gut. Furthermore, the immunohistochemical and Northern blot data presented by the authors do not prove that the observed differences in pAQP1 mRNA and protein abundance in porcine liver, small intestine, colon and salivary glands are due to differential and functional expression; the differential expression observed is more likely to be the consequence of the dissimilar vascularization of these tissues which will indisputably affect the abundance of AQP1-positive endothelial cells.
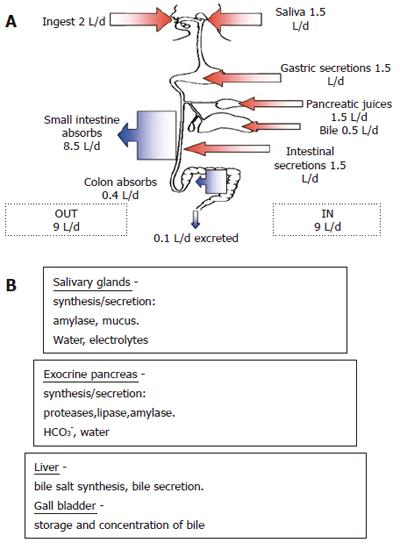
Based on the evidence from studies by other investigators, it is probably premature to suggest that AQP1 is involved in fluid secretion, fluid absorption, digestive function and pathophysiology of the porcine gastrointestinal system[1]. Multiple aquaporins are likely to be involved in the processes of fluid secretion and absorption in the digestive system. Aquaporins 3, 4, 8, 9 and 10 are also known to be expressed in strategic points along the gastrointestinal system[13]. The presence of several different AQP isoforms along the gastrointestinal tract is likely to be related to the specific water-transporting functions of each of its segments. For example, salivary glands synthesize salivary amylase and mucus which are secreted along with electrolytes and water. The exocrine pancreas synthesizes proteases, lipases and amylases which are secreted with HCO3- and water. The liver and gallbladder are involved in bile salt synthesis, its secretion, storage and concentration, respectively. Gastric parietal cells secrete HCl into the lumen of the stomach and hence require AQP water channels for their secretory function. Finally, the small and large intestines absorb up to 8.5 L and 0.4 L of water every day (Figure 2).
Figure 2 Summary of the gastrointestinal system’s daily fluid balance (A) and the secretions of salivary glands, exocrine pancreas and liver (B).
Studies on AQP-knockout mice have shown that some of the observed phenotypes are quite mild, especially in the gastrointestinal system which is not surprising given that water transport across the digestive epithelial barriers seems to occur not only via aquaporin water channels but also via other transporters, co-transporters or channel systems. The mild phenotypes of AQP-knockout mice also suggest that other aquaporins participate in compensatory mechanisms resulting from the selective disruption of a particular AQP gene.
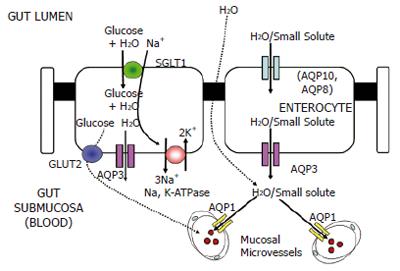
The anatomical localization and molecular identity of AQPs in the gastrointestinal tract is not particularly well studied compared to other organs, such as the kidney. There are very few published models of water and small solute transport across the epithelium of the gut. Accordingly, the focus of future studies should be identification and subcellular localization of AQP proteins in the mammalian (human) gut. We have recently proposed a model (Figure 3) of water and small solute transport across the epithelium of the human gut which incorporates the functional contribution of AQP1, AQP3, AQP8 and AQP10[21]. The data presented by Jin et al[1] also confirm our findings[23] and the findings of several other studies with regard to the distribution of the AQP1 protein in microvessel endothelia and bile ducts. However, further studies are still required to determine the expression of the recently identified members of the AQP family in the gastrointestinal system of humans in health and in diseases brought about by opportunistic bacteria (i.e., diarrhoea, colic and endotoxemia), immune disorders of the gut, such as Crohn’s disease and cancers of gastrointestinal organs. From a biological viewpoint, it would be of interest to perform comparative studies of AQP expression and function in gut tissues derived from mammals, amphibians, reptiles and fish. Also, determining the expression of AQP proteins in guts of omnivores, herbivores and carnivores will help us gain a more complete understanding of the functions of these proteins in the mammalian gastrointestinal system.
Figure 3 Model of water and small solute transport across the absorptive epithelium of the human small intestine[21].
The potential routes for water entry into the enterocyte from the luminal (apical) side are AQP10 and the sodium/glucose co-transporter SGLT1. SGLT1 is a secondary active transporter driven by the function of basolateral Na,K-ATPase which co-transports Na+ and glucose along with water into enterocytes. AQP8 (shown in parentheses) has been shown to be located in the sub-apical cytoplasm of absorptive epithelial cells. The basolateral water exit pathway is most likely to be the AQP3 protein[10,27]. Although the location of the AQP7 protein has not yet been precisely determined, it may be involved in glycerol transport in the intestinal lacteal system.