Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4425
Revised: March 28, 2006
Accepted: April 21, 2006
Published online: July 21, 2006
Melanoma accounts for 1-3 per cent of all malignant tumors. Except cutaneous, other less common melanomas include, among others, those in the GI tract. However, their primary or secondary nature is often difficult to establish. Referring to the stomach, scattered cases of primary melanomas have been reported in the literature.
We report a case of a man with an ulcerated sub-mucosal mass at the antrum of the stomach, manifested with dull upper abdominal pain, nausea, vomiting, fatigue and anemia. This lesion was histologically proved to be melanoma. A detailed clinical and laboratory investigation revealed no primary site elsewhere.
To our knowledge, very few cases of primary gastric melanoma have been reported. Our case is the fourth ever published and the first located at the antrum of the stomach. The debate upon the primitive nature of such lesions still persists. Thus, specific diagnostic criteria have been proposed.
- Citation: Lagoudianakis EE, Genetzakis M, Tsekouras DK, Papadima A, Kafiri G, Toutouzas K, Katergiannakis V, Manouras A. Primary gastric melanoma: A case report. World J Gastroenterol 2006; 12(27): 4425-4427
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4425
Primary gastrointestinal malignant melanoma is a very rare entity. In contrast, metastatic melanoma to the gas-trointestinal tract is found during diagnostic workup in 1%-4% of patients with a cutaneous primary and up to 60% of melanoma patients in autopsy[1-4]. Several reports have addressed the debate on whether primary gastrointestinal melanoma actually exists by proposing certain diagnostic criteria[5,6].
Primary melanomas located in the stomach are extremely rare. Although the cell of origin has not been identified since normal stomach epithelium lacks melanocytes, possible etiologies for the occurrence of primary melanoma have been described. Ectopic migration of melanocyte precursors or differentiation of the APUD cells to melanocytes has been advocated as possible mechanism for the development of malignant melanoma[7,8]. Furthermore, benign melanosis of the stomach has been reported in association with melanoma of the esophagus documenting the presence of melano-cytes in the stomach, rarely.
We report on a case of an ulcerated submucosal mass at the antrum of the stomach, which was histologically proved to be a melanoma. A detailed clinical and laboratory investigation revealed no primary site elsewhere.
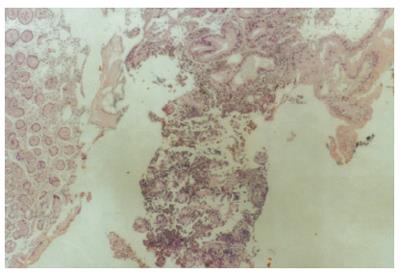
A 58-year old male was referred to our clinic with a short history of nonspecific symptoms, including nausea, vomiting, dull upper abdominal pain and fatigue, aggravating in intensity over the last 24 h. He had a weight loss of 8 kg over the preceding 3 mo. Clinical examination was unremarkable and laboratory examination showed anemia (Ht: 28%). A fecal occult blood test was positive. Colonoscopy was negative. Endoscopic examination of the upper GI tract revealed a submucosal mass with ulceration at the antrum, 3 cm × 5 cm in size. Biopsy, conducted during endoscopy, revealed a malignant melanotic lesion. Gastric mucosa proved to be infiltrated, by numerous pleomorphic tumor cells with melanin deposits (Figure 1).
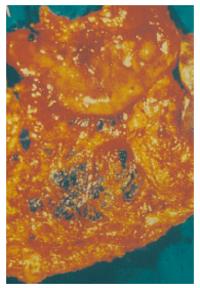
At exploratory laparotomy a melanotic lesion was found at the antrum with numerous melanotic lesions identified at the greater omentum (Figure 2). Subsequently, a subtotal gastrectomy with splenectomy was carried out.
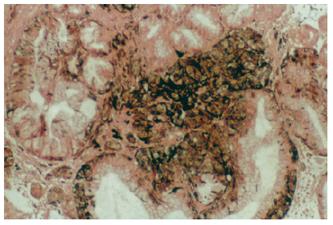
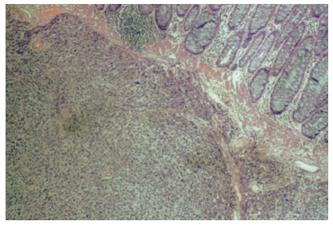
Pathological report on the surgical specimen proved the melanotic nature of the tumor. The gastric malignant melanoma was extending through the muscularis propria without invading the serosa. The excised lymph nodes were examined and found negative for metastases and complete excision of the ulcerated lesion was reported. Deposits of malignant melanocytes, accumulated after migrating from the antrum to the greater omentum through the extensive vascular and lymphatic vessels, were ascertained in the co-excised omentum. Microscopic evaluation showed nests and sheets of epithelioid cells stained positive with Fontana-Masson (Figure 3). Immunohistochemical examination revealed a positive reaction with S-100 protein and HMB-45 antibodies (Figure 4).
The postoperative clinical investigation, as well as the follow-up did not reveal any other primary site. Ophthalmologic and dermatologic examinations were negative. In addition, anoscopy, chest CT and small-bowel barium contrast radiography were also negative for a primary site of melanoma.
The patient denied any further adjuvant therapy. Sixteen months after the operation he is disease-free, as follow-up endoscopic examination of the upper GI tract and abdominal CT revealed no signs of recurrence.
Non-cutaneous melanoma represents a rare form of melanoma. In a review of 84 836 cases of melanoma between 1985 and 1994, 91.2% were cutaneous, 5.2% ocular, 1.3% mucosal and 2.2% of unknown primary[9]. Referring to GI mucosal sites, melanoma has been reported to arise in esophagus, anorectum and small intestine[10-12]. Moreover, scattered cases of primary melanoma arising in the stomach have also been reported[5,6,13].
The proposed primary sources, from which GI melanomas are derived, are melanoblastic cells of the neural crest, which migrate to the ileum through the omphalomesenteric canal or APUD cells, which undergo neoplastic transformation[14]. Although, there have been no reports on benign melanocytes in normal gastric epithelium, melanosis of the gastric mucosa in association with esophageal and anal malignant melanoma has been reported[15]. Furthermore, the rare presence of melanosis of the esophageal mucosa is accounted for the occurrence of primary esophageal melanoma[16]. Thus, it seems reasonable that primary gastric melanoma should be considered possible.
In order to avoid running the risk of misinterpretation of a metastasis to the gastric wall to primary lesion, several reports have issued criteria for the diagnosis of primary melanoma. Suggestive criteria of the primary nature of gastric melanoma include lack of concurrent or previous removal of a melanoma or atypical melanotic lesion from the skin, lack of other organ involvement and in-situ change in the overlying or adjacent GI epithelium. The latter, recognized histologically by the presence of atypical melanotic cells in the basal layer of the epithelium and extending in a “pagetoid” fashion into the more superficial epithelium, may be reported in 40%-100% of primary GI melanomas[14,17]. Additionally, disease free survival of at least 12 mo after curative surgical excision of the involved organ has been proposed as a criterion for the distinction of a primary lesion from metastatic since 50% of patients with stage IV melanoma or visceral disease from unknown primary will have died at 12 mo from diagnosis[9,10].
Etiology of primary GI melanomas remains undefined. No predisposing factor has ever been proposed. In contrast, the rarity of these lesions is easily justified by the paucity of melanocytes in the gastrointestinal track and the inherent protection from etiologic factors such as ultraviolet radiation[10].
Manifestations of primary gastric melanoma include anemia and weight loss. Barium contrast radiography and endoscopy have some role in the initial diagnostic procedure. CT scan of the abdomen may reveal a gastric mass with or without evidence of lymph node metastases. Preoperative diagnosis is seldom made because of the non-specificity of the symptoms, including nausea, vomiting, abdominal pain, weight loss and anemia[18]. Tissue sampling during endoscopy may provide the definitive diagnosis. The presence of pigmentation of an ulcer is the most common endoscopic finding. Immunohistochemical staining with HMB-45 and S-100 will confirm the presence of malignant melanocytes in the mucosa[19].
Melanomas that arise on mucosal surfaces appear to be more aggressive and are associated with worse prognosis than cutaneous melanomas. The poorer prognosis may be related to delay in diagnosis, an inherently more aggressive behavior of mucosal melanomas or earlier dissemination because of the rich lymphatic and vascular supply of the GI tract mucosa[10]. However, early detection of primary gastrointestinal melanomas and treatment with curative surgical excision can provide long-term disease free interval[13].
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
| 1. | Frost DB, Mercado PD, Tyrell JS. Small bowel cancer: a 30-year review. Ann Surg Oncol. 1994;1:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Reintgen DS, Thompson W, Garbutt J, Seigler HF. Radiologic, endoscopic, and surgical considerations of melanoma metastatic to the gastrointestinal tract. Surgery. 1984;95:635-639. [PubMed] |
| 3. | Backman H. Metastases of malignant melanoma in the gastrointestinal tract. Geriatrics. 1969;24:112-120. [PubMed] |
| 4. | Ihde JK, Coit DG. Melanoma metastatic to stomach, small bowel, or colon. Am J Surg. 1991;162:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Chandler AB, Jones GF. Malignant melanoma of the gastrointestinal tract; a case report. Am Surg. 1951;17:719-721. [PubMed] |
| 6. | Macák J. Melanoma of the stomach: reality or fiction. Pathologica. 1998;90:388-390. [PubMed] |
| 7. | Tabaie HA, Citta RJ, Gallo L, Biondi RJ, Meoli FG, Silverman D. Primary malignant melanoma of the small intestine: report of a case and discussion of the APUD cell concept. J Am Osteopath Assoc. 1984;83:374-377. [PubMed] |
| 8. | Krausz MM, Ariel I, Behar AJ. Primary malignant melanoma of the small intestine and the APUD cell concept. J Surg Oncol. 1978;10:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Sachs DL, Lowe L, Chang AE, Carson E, Johnson TM. Do primary small intestinal melanomas exist Report of a case. J Am Acad Dermatol. 1999;41:1042-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Chalkiadakis G, Wihlm JM, Morand G, Weill-Bousson M, Witz JP. Primary malignant melanoma of the esophagus. Ann Thorac Surg. 1985;39:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Kadivar TF, Vanek VW, Krishnan EU. Primary malignant melanoma of the small bowel: a case study. Am Surg. 1992;58:418-422. [PubMed] |
| 13. | Alazmi WM, Nehme OS, Regalado JJ, Rogers AI. Primary gastric melanoma presenting as a nonhealing ulcer. Gastrointest Endosc. 2003;57:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Elsayed AM, Albahra M, Nzeako UC, Sobin LH. Malignant melanomas in the small intestine: a study of 103 patients. Am J Gastroenterol. 1996;91:1001-1006. [PubMed] |
| 15. | Horowitz M, Nobrega MM. Primary anal melanoma associated with melanosis of the upper gastrointestinal tract. Endoscopy. 1998;30:662-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Germano D, Rosati G, Romano R, Vita G, Lepore G, De Sanctis D, Manzione L. Primary gastric melanoma presenting as a double ulcer. J Clin Gastroenterol. 2004;38:828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Christova S, Meinhard K, Mihailov I, Alexiev B. Three cases of primary malignant melanoma of the alimentary tract. Gen Diagn Pathol. 1996;142:63-67. [PubMed] |
| 18. | Blecker D, Abraham S, Furth EE, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427-3433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Clemmensen OJ, Fenger C. Melanocytes in the anal canal epithelium. Histopathology. 1991;18:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |