Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4331
Revised: March 8, 2006
Accepted: March 27, 2006
Published online: July 21, 2006
AIM: To clarify the mechanism underlying the anti-diabetic activities of cortex cinnamomi extract (CCE).
METHODS: To induce in vivo diabetes, mice were injected with streptozotocin (STZ) via a tail vein (100 mg STZ/kg body weight). To determine the effects of CCE, mice were administered CCE twice daily for 7 d by oral gavage starting 1 wk before the STZ injection. Blood glucose and plasma insulin concentration were measured as an index of diabetes. Also, to induce cytotoxicity of RINm5F cells, we treated with cytokines (IL-1β (2.0 ng/mL) and IFN-γ (100 U/mL)). Cell viability and nitric oxide production were measured colorimetrically. Inducible nitric oxide synthase (iNOS) mRNA and protein expression were determined by RT-PCR and Western blotting, respectively. The activation of NF-κB was assayed by using gel mobility shift assays of nuclear extracts.
RESULTS: Treatment of mice with STZ resulted in hyperglycemia and hypoinsulinemia, which was further evidenced by immunohistochemical staining of islets. However, the diabetogenic effects of STZ were completely prevented when mice were pretreated with CCE. The inhibitory effect of CCE on STZ-induced hyperglycemia was mediated through the suppression of iNOS expression. In rat insulinoma RINm5F cells, CCE completely protected against interleukin-1β and interferon-γ-mediated cytotoxicity. Moreover, RINm5F cells incubated with CCE showed significant reductions in interleukin-1β and interferon-γ-induced nitric oxide production and in iNOS mRNA and protein expression, and these findings correlated well with in vivo observations.
CONCLUSION: The molecular mechanism by which CCE inhibits iNOS gene expression appears to involve the inhibition of NF-κB activation. These results reveal the possible therapeutic value of CCE for the prevention of diabetes mellitus progression.
-
Citation: Kwon KB, Kim EK, Jeong ES, Lee YH, Lee YR, Park JW, Ryu DG, Park BH.
Cortex cinnamomi extract prevents streptozotocin- and cytokine-induced β-cell damage by inhibiting NF-κB. World J Gastroenterol 2006; 12(27): 4331-4337 - URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4331.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4331
Insulin-dependent diabetes mellitus (IDDM) is an autoimmune disease that results from the selective destruction of pancreatic β-cells[1]. At earlier stages of the disease, histological findings show features of insulitis characterized by the infiltration of immune cells, such as T lymphocytes, macrophages, and natural killer cells into pancreatic islets. These cells produce and release various cytokines which act as humoral mediators of the immunologic process. Thus, cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α, and interferon-γ (IFN-γ) have been implicated as key effector molecules in β-cell function and viability[2,3].
Alloxan and streptozotocin (STZ) are two commonly used diabetogenic agents. These structurally diverse compounds have a long history of use in diabetes research and are known to be specifically toxic to pancreatic β-cells. Alloxan is thought to produce oxygen free radicals, whereas STZ releases nitric oxide (NO) and oxygen free radicals during its metabolism[4-8].
The biochemical mechanisms that mediate the detrimental effects of cytokines and streptozotocin remain elusive. Recent studies suggest that oxygen free radicals or NO mediates the deleterious effects of cytokine or streptozotocin on β-cell dysfunction and destruction[9,10]. NO is produced by the oxidation of L-arginine to L-citruline by nitric oxide synthase (NOS)[11], and excess NO generated in cells may inhibit mitochondrial metabolism, protein modification, and DNA cleavage, any one of which could lead to insulin secretion impairment and β-cell death [9,12,13].
The induction of iNOS is regulated by transcription factors that bind to specific sites in the promoter of the iNOS gene. It is known that the activation of the transcription factor NF-κB, which can be induced by various stimuli, e.g., IL-1β, INF-γ, bacterial lipopolysaccharide and streptozotocin, is essentially required for iNOS expression[14,15]. NF-κB is initially located in the cytoplasm as an inactive form complexed with IκB, an NF-κB inhibitory factor. Various inducers cause this dissociation of this complex presumably via the phosphorylation of IκB, and allow NF-κB to be released from the complex. NF-κB then translocates to the nucleus, where it interacts with its DNA recognition sites to mediate gene transcription[16]. In light of the possible role of NO in the pathogenesis of autoimmune diabetes, we[17,18] and others[19-21] have previously shown that NF-κB-dependent NO production plays a key role in the dysfunction and destruction of β-cells.
Cortex cinnamomi (CC) is the name given to the bark of Cinnamomum cassia Presl, which belongs to the Lauraceae family, and is commonly used in traditional Chinese medicine for treating dyspepsia, gastritis, blood circulation disturbances, and inflammatory disease. Moreover, it has been reported that CC extract (CCE) has anti-inflammatory activity and an anti-thrombic effect[22,23]. In the present study, we observed the preventive effects of CCE on cytokine- and STZ-induced pancreatic β-cell damage both in vitro and in vivo. CCE was found to prevent NF-κB activation, iNOS mRNA and protein expression, and subsequent NO production, and thus protected RINm5F cells and pancreas from cytokine and STZ induced damage.
Specific pathogen-free female ICR mice were purchased from the Korean Research Institute of Chemical Technology (Daejon, Korea) and housed at our animal facility for one week before use. All mice used were 5-6 wk old, and were kept under specific pathogen-free conditions with free access to a standard commercial diet.
To induce diabetes, mice were injected with STZ via a tail vein (100 mg STZ/kg body weight). STZ was dissolved in 0.1 mol/L sodium citrate buffer (pH 4.0) and injected within 5 min. To determine the effects of CCE, mice were divided into the following groups; 1) the non-treated control group, 2) the STZ group, 3) the CCE (100 mg/kg) + STZ group, 4) the CCE (250 mg/kg) + STZ group, and the 5) CCE (500 mg/kg) + STZ group (n = 7, each group). Mice were administered CCE twice daily for 7 d by oral gavage starting 1 wk before the STZ injection. The day of injection was defined as d 1. Control group animals were administered citrate buffer alone, i.e., they were not treated with STZ or CCE. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Wonkwang University, Oriental Medical School. On the day of sacrifice, mice were decapitated without anesthesia and trunk blood was collected into prechilled tubes containing 1 mg/mL of EDTA for insulin and glucose determinations.
RINm5F is an insulinoma cell line that was derived from a rat islet cell tumor[24]. Cells were purchased from the American Type Culture Collection and grown at 37°C in a humidified 50 mL/L CO2 atmosphere in RPMI 1640 medium (Gibco BRL) supplemented with 10% fetal bovine serum and 2 mmol/L glutamine, 10000 units/mL of penicillin, 10 mg/mL of streptomycin, and 2.5 μg/mL of amphotericin B.
The herb was identified as Cortex cinnamomi (CC) by local experts. Voucher samples are preserved for reference in the Herbarium of the Department of Physiology, School of Oriental Medicine, Wonkwang University (ref. Omcphy, 2001-05). For extraction, 200 g of dried CC was added to 1800 mL of water and boiled for 2 h, filtered, and concentrated to 200 mL. The sterile extract (7.03 g) so obtained was stored at -20°C.
The viabilities of cultured cells were determined by using MTT assays, which involve the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan as described previously[25]. In brief, after 24 h of incubation, cells (1 × 105/well) in 96-well plates were washed twice with PBS, and MTT (100 μg/0.1 mL of PBS) was added to each well. Cells were then incubated at 37°C for 1 h, and DMSO (100 μL) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a model Spectra MAX PLUS (Molecular Devices, Sunnyvale, CA).
Blood glucose was determined using a glucose oxidase kit (Asan Pharm. Co., Korea), and plasma insulin was assayed using a standard radioimmunoassay technique (Linco Research Immunoassay, St. Charles, MO).
Biologically produced NO is rapidly oxidized to nitrite and nitrate in aqueous solutions[26], and thus, nitrite concentrations in cell-free culture supernatant reflect NO production, and may be measured colorimetrically[27]. Following incubation for 24 h, 100 μL aliquots of culture supernatants were incubated with 100 μL of a 1:1 mixture of 10 g/L sulfanilamide in 300 g/L acetic acid and 1 g/L N-(1-naphthyl) ethylenediamine dihydrochloride in 600 g/L acetic acid at room temperature. After 5 min, absorbances were measured at 540 nm using a spectrophotometer. Concentrations were determined using a linear standard curve drawn using serial dilutions of sodium nitrite in working medium.
Cells and pancreases were homogenized in 100 μL of ice-cold lysis buffer (20 mmol/L HEPES, pH 7.2, 10 g/L Triton X-100, 100 g/L glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin). Homogenates containing 20 μg of protein were separated by SDS-PAGE with 100 g/L resolving and 30 g/L acrylamide staking gel, and transferred to nitrocellulose sheets (Millipore, Bedford, MA). These were then blocked with 20 g/L bovine serum albumin and incubated for 4 h with 1 μg/mL anti-mouse macrophage iNOS antibody (Transduction Lab, Lexongton, KY). iNOS protein expression levels were determined using a Chemi-doc image analyzer (Bio-Rad, Hercules, CA).
Total RNA was isolated from cells and pancreases using TRIzol from Invitrogen (Carlsbad, CA), as instructed by the manufacturer. One microgram of total RNA was transcribed into cDNA in a 20 μL final volume of reaction buffer (10 mmol/L Tris-HCl, pH 7.4, 50 mmol/L MgCl2, 1 mmol/L each dNTP) and 2.4 μmol/L oligo-d(T)16-primer, 1 unit RNase inhibitor, and 2.5 units MulV reverse transcriptase by incubation for 10 min at 21°C and 15 min at 42°C. The reaction was stopped by incubation at 99°Cfor 5 min. For rat iNOS PCR, aliquots of the synthesized cDNA were added to a 45 μL PCR mixture containing 10 mmol/L Tris-HCl, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L of each dNTP, 2 units Taq DNA polymerase, and 0.4 μmol/L of each PCR primer; iNOS upstream primer, 5’-CCACAATAGTACAATACTACTTGG-3’, down stream primer, 5’-ACGAGGTGTTCAGCGTGCTCCACG-3’. Amplification was initiated by 3 min of denaturation at 94°C, and amplification was performed using 26 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min and was followed by a single extension step of 5 min at 72°C. β-actin PCR was performed using a 2.5 μL aliquot of synthesized cDNA using primers at a concentration of 0.15 μmol/L; β-actin upstream primer, 5’-TGCCCATCTATGAGGGTTACG-3’ down stream primer, TAGAAGCATTTGCGGTGCACG-3’. The obtained PCR products were analyzed on ethidium bromide-stained agarose (15 g/L) gels.
Nuclear extracts were prepared as described previously[18]. Cells and pancreases were immediately washed twice, scraped into 1.5 mmol/L of ice-cold PBS (pH 7.9), and pelleted at 12 000 g for 30 s. Cell pellets were suspended in ice-cold hypotonic lysis buffer (10 mmol/L HEPES, 1.5 mmol/L MgCl2, 0.2 mmol/L KCl, 0.2 mmol/L phenylmethylsulphonylfluoride, 0.5 mmol/L dithiothreitol), vortexed for 10 sec, and then centrifuged at 3000 r/min for 5 min. Cells pellets were resuspended with ice-cold hypotonic lysis buffer in the presence of 50 μL of 100 g/L Nonidet P-40 and then incubated on ice for 25 min. Nuclear fractions were precipitated by centrifugation at 4000 rpm for 15 min, pellets were resuspended in 50-100 μL of low salt extraction buffer (20 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 250 g/L glycerol, 20 mmol/L KCl, 0.2 mmol/L EDTA, 0.2 mmol/L phenylmethylsulphonylfluoride, 0.5 mmol/L dithiothreitol) and added dropwise to equal volumes of high salt extraction buffer (20 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 250 g/L glycerol, 80 mmol/L KCl, 0.2 mmol/L EDTA, 0.2 mmol/L phenylmethylsulphonylfluoride, 0.5 mmol/L dithiothreitol). Mixtures were then incubated with continuous shaking at 4°Cfor 45 min, centrifuged for 20 min at 12 000 g, and nuclear extracts were aliquoted and stored at -80°C. Protein concentrations were determined using the method of Bradford[28].
The activation of NF-κB was assayed by using gel mobility shift assays of nuclear extracts from control and treated cells[18]. As a probe for gel retardation assays, an oligonucleotide containing the κ-chain binding site (κB, 5'-CCGGTTAACAGAGGGGGCTTTCCGAG-3') was synthesized and labeled with [α-32P]dCTP. Labeled oligonucleotides (10 000 cpm), 10 μg of nuclear extracts, and binding buffer (10 mmol/L Tris-HCl, pH 7.6, 500 mmol/L KCl, 10 mmol/L EDTA, 500 g/L glycerol, 100 ng poly (dI.dC), 1mmol/L DTT) were incubated for 30 min at room temperature in final volume of 20 μL. Reaction mixtures were analyzed by electrophoresis in 40 g/L polyacrylamide gels in 0.5 × Tris-borate buffer. Gels were then dried and autoradiographed. Specific binding was controlled by competition with a 50-fold excess of cold κB oligonucleotide.
Pancreases removed under anesthesia were immediately placed in fixative (40 g/L formaldehyde solution in 0.1 mol/L PBS) overnight, and washed with 0.1 mol/L PBS. To account for variations between pancreatic regions, tissues were cut into 4-mm blocks, which were then randomly inserted into a cassette and glycol methacrylate (GMA, Technovit 8100) embedded, sectioned (2 μm), and stained with haematoxylin and eosin (H-E). For immunostaining studies, guinea pig anti-swine insulin antibody (working dilution 1:50; DakoCytomation, Belgium) was incubated with sections for 3 h at 37°C. FITC-conjugated goat anti-guinea pig IgG antibody (working dilution 1:25, Jackson ImmunoResearch Laboratories. Inc, West Grove, PA) was used for fluorescence imaging.
Statistical analysis was performed using the Student’s t-test and ANOVA. P values of < 0.05 were considered statistically significant.
Mice that received 100 mg/kg of STZ became hyperglycemic at 72 h. Their blood glucose levels at 72 h were 16.43 ± 2.16 mmol/L, a value within the acceptable diabetic range. In contrast, the mice pretreated with CCE and treated with STZ showed normal blood glucose values (Table 1). Moreover, this glucose lowering effect of CCE was dose-dependent. CCE treatment alone did not affect blood glucose values in mice (data not shown). Animals injected with STZ alone showed significant decreases in plasma insulin levels (0.06 ± 0.01 ng/mL) versus non-treated controls (0.21 ± 0.04 ng/mL). Pretreatment of mice with CCE attenuated the severity of STZ-induced hypoinsulinemia (Table 1). Thus, CCE was protective against STZ-induced diabetes.
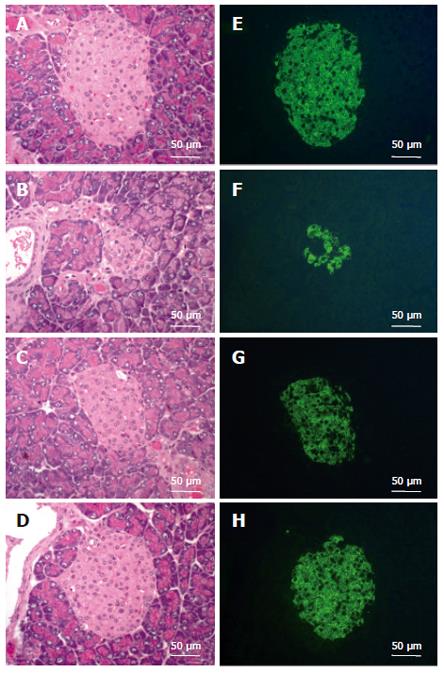
To elucidate the preventive effect of CCE on STZ-induced diabetes, pancreatic islets were histologically examined. Pancreatic tissues were obtained one week after STZ administration with or without CCE pretreatment and subjected to H-E staining and immunohistochemistry. In diabetic mice not treated with CCE, the most consistent findings in pancreatic sections stained with H-E were degenerative and necrotic changes, and islet shrinkage (Figure 1B). Immunohistochemical staining showed weak insulin-reactivity in a few β-cells (Figure 1F). However, diabetic mice pretreated with CCE showed near normal islets by both H-E staining and immunohistochemistry; i.e., round shaped and clearly defined islets with strong insulin positive staining (Figures 1C, 1D, 1G and 1H).
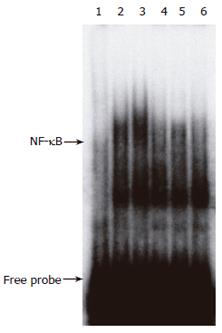
To clarify the antidiabetogenic mechanism of CCE, we examined its effect on STZ-induced NF-κB activation. The findings of this investigation are in accord with those of previous studies, which demonstrated that in mice, STZ treatment results in NF-κB activation[10]. Figure 2 shows a representative EMSA radiograph showing the 32P-DNA/NF-κB complex in nuclear extracts of pancreas 30 min after STZ injection. In contrast, this complex was not detected in nuclear extracts from CCE-pretreated mice. This result shows that CCE inhibits the translocation of NF-κB to the nucleus in mice.
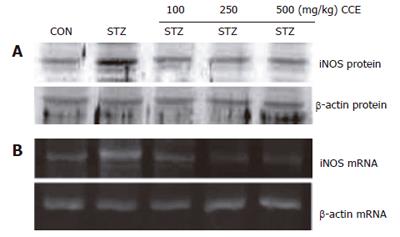
iNOS is a downstream target of NF-κB activation. Thus, we examined whether CCE modulates the induction of iNOS protein after STZ injection (Figure 3A). STZ injected mice expressed high levels of iNOS protein. However, this STZ-induced upregulation of iNOS protein was totally suppressed by pretreating with CCE. We examined iNOS steady state mRNA levels after STZ injection by RT-PCR (Figure 3B). iNOS mRNA was found to be induced 24 h after STZ injection, whereas no iNOS mRNA was detected in pancreases of CCE-pretreated mice. The results suggest that iNOS, a down stream target of NF-κB, is induced by STZ injection, and that CCE pretreatment inhibits this induction, possibly by inhibiting NF-κB.
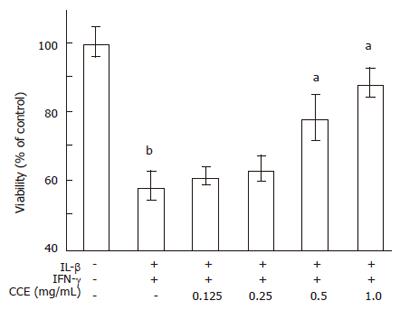
We next investigated the anti-diabetogenic effect of CCE at the cellular level. RINm5F cells, a rat pancreatic β-cell line, were cultured to near confluence, and treated with a high dose combination of cytokines (IL-1β (2.0 ng/mL) and IFN-γ (100 U/mL)) with/without CCE for 24 h. Percentages of viable cells were detected using MTT assays. As shown in Figure 4, cytokine-treated cells had a viability of 58.2% ± 4.5%, whereas cells pretreated with CCE (0.5 mg/mL) had a viability of 78.2% ± 6.8%. Moreover, a two-fold higher dose of CCE (1.0 mg/mL) increased cell viability to 88.3 ± 4.2%. CCE alone did not affect the cell viability (data not shown).
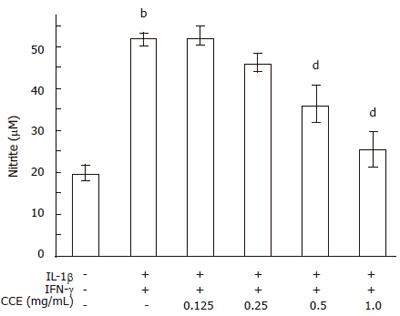
NO production was evaluated using the same conditions. Control RINm5F cells generated 19.8 ± 2.0 μmole/L of nitrite, whereas cytokine treated cells generated 51.8 ± 1.6 μmole/L of nitrite in 24 h (Figure 5). On the other hand, RINm5F cells treated with cytokines plus CCE showed concentration dependent reductions in cytokine-mediated nitrite production. Near complete inhibition of nitrite production was observed at a CCE concentration of 1.0 mg/mL. Treatment with CCE alone did not produce nitrite (data not shown).
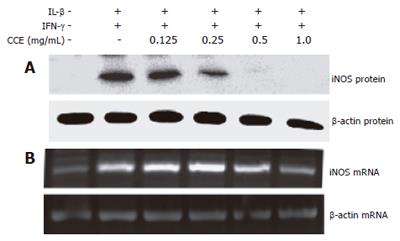
To investigate the regulatory effect of CCE on NO production, we examined the effects of CCE on cytokine-induced iNOS mRNA and protein expressions using RT-PCR and Western blotting, respectively. As shown in Figure 6, the above cytokine combination increased iNOS mRNA and protein levels. However, when CCE was added to the cytokine mixture, iNOS mRNA and protein levels reduced dose-dependently, though 0.125 mg/mL CCE was ineffective, 1.0 mg/mL of CCE completely blocked iNOS mRNA expression.
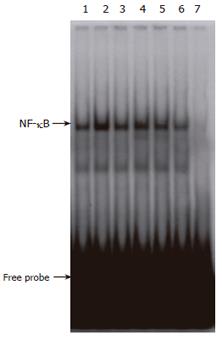
Because NF-κB has been implicated in the toxicity of STZ (Figure 2), we studied the effect of CCE on the cytokine-stimulated translocation of NF-κB from the cytosol to the nucleus in RINm5F cells. Nuclear extracts from cytokine-stimulated RINm5F cells showed an increase in NF-κB binding activity (Figure 7, lane 2), and this was markedly suppressed by adding CCE, implying that CCE inhibits iNOS expression and NO production by inhibiting NF-κB activation in RINm5F cells. The specificity of the DNA-NF-κB interaction was demonstrated by competitive assays using a 50-fold excess of unlabeled oligonucleotide (Figure 7, lane 7). These results indicate that the cytokine-induced expression of iNOS protein in RINm5F cells is inhibited by CCE at the transcriptional level.
IDDM is an autoimmune disease characterized by the specific destruction of β-cells in the islets of Langerhans[1]. Cumulative evidence suggest that NF-κB dependent NO production is a critical component of the destruction and dysfunction of β-cells. NF-κB activation in β-cells can be triggered by cytokines and by diabetogenic drugs such as STZ and alloxan[10,14,15]. Therefore, reduced NO production presents a potential means of overcoming β-cell failure.
The use of traditional plant medicines has been practiced for centuries by mankind, and despite a general insufficiency of supportive evidence concerning therapeutic efficacies, herbal medicine usage continues to increase. The present results confirm previous observations that cytokines and STZ induce NF-κB activation, iNOS expression, NO production, and β-cell dysfunction in both mice pancreases and cultured β-cells, and that CCE abolishes cytokine- and STZ-mediated β-cell damage by blocking NF-κB translocation to the nucleus. Thereby, these results indicate that CCE can preserve the insulin secreting capacity and viability of β-cells.
In the first series of experiments, we used mice to observe NF-κB activation by STZ and its prevention by CCE. EMSA studies revealed increased NF-κB binding activity in pancreatic nuclear extracts derived from STZ-treated hyperglycemic diabetic mice. In contrast, pretreatment with CCE prevented NF-κB activation and iNOS expression, and this resulted in the maintenance of plasma glucose and insulin levels in the normal range. To further confirm the preventive effect of CCE, histological examinations on pancreatic islets were performed. STZ-treated mice showed marked islet destruction and relative small numbers of insulin-positive β-cells, whereas well-defined islets and strong insulin positive staining were observed in CCE pre-treated mice. STZ-induced diabetic rodents were previously reported to show increased iNOS activity and expression in islets at a very early stage[4,5,8]. In this situation, NO generated may react with oxygen free radicals like the superoxide anion, to form highly oxidizing peroxynitrite, which would lead to more aggressive oxidative and nitrosative stress.
Similar effects of CCE were observed in RINm5F cells exposed to cytokines for 24 h. The reduction in NO production induced by CCE in RINm5F cells was associated with a reduction in NF-κB activation and iNOS mRNA expression. These findings are in line with recent observations that the extract of A. xanthoides reduces iNOS expression and protects β-cells in mice and RINm5F cells from alloxan[17,18]. Future studies are needed to determine the target pathway that leads to the sequestration of NF-κB in the cytosol.
The generation of oxygen free radicals is another important mechanism of cytokine- and other diabetogenic drug-mediated toxicities in β-cells. Experimental evidence indicates that oxygen free radicals are generated in both cytokine-stimulated[6,29,30] and STZ-treated β-cells[31], and that the overexpression of antioxidant enzymes protects β-cells from such insults[7,32,33]. Furthermore, oxygen free radicals induce NF-κB activation and iNOS expression in rodent and human β-cells[34,35], which suggests a role for hydrogen peroxide in the pathway of NF-κB activation and iNOS expression induced by cytokines. We cannot exclude the possibility that the protective effect of CCE occurs via the reduced generation of oxygen free radicals.
In summary, our results indicate that CCE inhibits cytokines- and STZ-induced β-cell damage in vivo and in vitro. The primary mechanism underlying this effect is the inhibition of iNOS protein expression, which may be mediated at the transcriptional level through the inhibition of NF-κB activation and iNOS transcription. These findings suggest that CCE is likely to have a beneficial effect when used to prevent or attenuate the progress of diabetes mellitus.
S- Editor Wang J L- Editor Romero-Gomez M E- Editor Liu Y
| 1. | Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 616] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 2. | Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 381] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab. 1994;79:1058-1062. [PubMed] [DOI] [Full Text] |
| 4. | Corbett JA, Mikhael A, Shimizu J, Frederick K, Misko TP, McDaniel ML, Kanagawa O, Unanue ER. Nitric oxide production in islets from nonobese diabetic mice: aminoguanidine-sensitive and -resistant stages in the immunological diabetic process. Proc Natl Acad Sci U S A. 1993;90:8992-8995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Lindsay RM, Smith W, Rossiter SP, McIntyre MA, Williams BC, Baird JD. N omega-nitro-L-arginine methyl ester reduces the incidence of IDDM in BB/E rats. Diabetes. 1995;44:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Takasu N, Asawa T, Komiya I, Nagasawa Y, Yamada T. Alloxan-induced DNA strand breaks in pancreatic islets. Evidence for H2O2 as an intermediate. J Biol Chem. 1991;266:2112-2114. [PubMed] |
| 7. | Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 715] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 8. | Wu G. Nitric oxide synthesis and the effect of aminoguanidine and NG-monomethyl-L-arginine on the onset of diabetes in the spontaneously diabetic BB rat. Diabetes. 1995;44:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Corbett JA, McDaniel ML. Does nitric oxide mediate autoimmune destruction of beta-cells Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Ho E, Chen G, Bray TM. Alpha-phenyl-tert-butylnitrone (PBN) inhibits NFkappaB activation offering protection against chemically induced diabetes. Free Radic Biol Med. 2000;28:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Eizirik DL, Delaney CA, Green MH, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerström C, Green IC. Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol. 1996;118:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kaneto H, Fujii J, Seo HG, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 214] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Stadler J, Billiar TR, Curran RD, Stuehr DJ, Ochoa JB, Simmons RL. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991;260:C910-C916. [PubMed] |
| 14. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3586] [Article Influence: 128.1] [Reference Citation Analysis (0)] |
| 15. | Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 1999;13:1845-1854. [PubMed] |
| 16. | Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3640] [Article Influence: 117.4] [Reference Citation Analysis (1)] |
| 17. | Kwon KB, Kim JH, Lee YR, Lee HY, Jeong YJ, Rho HW, Ryu DG, Park JW, Park BH. Amomum xanthoides extract prevents cytokine-induced cell death of RINm5F cells through the inhibition of nitric oxide formation. Life Sci. 2003;73:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Park BH, Park JW. The protective effect of Amomum xanthoides extract against alloxan-induced diabetes through the suppression of NFkappaB activation. Exp Mol Med. 2001;33:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kim MJ, Ryu GR, Kang JH, Sim SS, Min DS, Rhie DJ, Yoon SH, Hahn SJ, Jeong IK, Hong KJ. Inhibitory effects of epicatechin on interleukin-1beta-induced inducible nitric oxide synthase expression in RINm5F cells and rat pancreatic islets by down-regulation of NF-kappaB activation. Biochem Pharmacol. 2004;68:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kwon G, Hill JR, Corbett JA, McDaniel ML. Effects of aspirin on nitric oxide formation and de novo protein synthesis by RINm5F cells and rat islets. Mol Pharmacol. 1997;52:398-405. [PubMed] |
| 21. | Mandrup-Poulsen T, Helqvist S, Wogensen LD, Mølvig J, Pociot F, Johannesen J, Nerup J. Cytokine and free radicals as effector molecules in the destruction of pancreatic beta cells. Curr Top Microbiol Immunol. 1990;164:169-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Matsuda H, Matsuda R, Fukuda S, Shiomoto H, Kubo M. Anti-thrombic actions of 70% methanolic extract and cinnamic aldehyde from cinnamomi cortex. Chem Pharm Bull (Tokyo). 1987;35:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Kubo M, Ma S, Wu J, Matsuda H. Anti-inflammatory activities of 70% methanolic extract from Cinnamomi Cortex. Biol Pharm Bull. 1996;19:1041-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gazdar AF, Chick WL, Oie HK, Sims HL, King DL, Weir GC, Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980;77:3519-3523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 331] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Kwon KB, Yang JY, Ryu DG, Rho HW, Kim JS, Park JW, Kim HR, Park BH. Vibrio vulnificus cytolysin induces superoxide anion-initiated apoptotic signaling pathway in human ECV304 cells. J Biol Chem. 2001;276:47518-47523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 27. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9026] [Article Influence: 209.9] [Reference Citation Analysis (0)] |
| 28. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157317] [Article Influence: 3210.6] [Reference Citation Analysis (0)] |
| 29. | Suarez-Pinzon WL, Szabó C, Rabinovitch A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes. 1997;46:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Sumoski W, Baquerizo H, Rabinovitch A. Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia. 1989;32:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 636] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 32. | Lortz S, Tiedge M, Nachtwey T, Karlsen AE, Nerup J, Lenzen S. Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes. 2000;49:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Tiedge M, Lortz S, Munday R, Lenzen S. Protection against the co-operative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia. 1999;42:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Bedoya FJ, Flodström M, Eizirik DL. Pyrrolidine dithiocarbamate prevents IL-1-induced nitric oxide synthase mRNA, but not superoxide dismutase mRNA, in insulin producing cells. Biochem Biophys Res Commun. 1995;210:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Flodström M, Niemann A, Bedoya FJ, Morris SM Jr, Eizirik DL. Expression of the citrulline-nitric oxide cycle in rodent and human pancreatic beta-cells: induction of argininosuccinate synthetase by cytokines. Endocrinology. 1995;136:3200-3206. [PubMed] [DOI] [Full Text] |