Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4296
Revised: July 28, 2005
Accepted: December 25, 2005
Published online: July 21, 2006
AIM: To study the association between nitrite and nitrosamine intake and gastric cancer (GC), between meat and processed meat intake, GC and oesophageal cancer (OC), and between preserved fish, vegetable and smoked food intake and GC.
METHODS: In this article we reviewed all the published cohort and case-control studies from 1985-2005, and analyzed the relationship between nitrosamine and nitrite intake and the most important related food intake (meat and processed meat, preserved vegetables and fish, smoked foods and beer drinking) and GC or OC risk. Sixty-one studies, 11 cohorts and 50 case-control studies were included.
RESULTS: Evidence from case-control studies supported an association between nitrite and nitrosamine intake with GC but evidence was insufficient in relation to OC. A high proportion of case-control studies found a positive association with meat intake for both tumours (11 of 16 studies on GC and 11 of 18 studies on OC). A relatively large number of case-control studies showed quite consistent results supporting a positive association between processed meat intake and GC and OC risk (10 of 14 studies on GC and 8 of 9 studies on OC). Almost all the case-control studies found a positive and significant association between preserved fish, vegetable and smoked food intake and GC. The evidence regarding OC was more limited. Overall the evidence from cohort studies was insufficient or more inconsistent than that from case-control studies.
CONCLUSION: The available evidence supports a positive association between nitrite and nitrosamine intake and GC, between meat and processed meat intake and GC and OC, and between preserved fish, vegetable and smoked food intake and GC, but is not conclusive.
- Citation: Jakszyn P, González CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J Gastroenterol 2006; 12(27): 4296-4303
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4296
Humans are exposed to a wide range of N-nitroso-compounds (NOCs) from diet, tobacco smoking, work place and drinking water[1,2], which are the major source of exposure in the general population[3]. Preformed exogenous nitrosamines are found mainly in cured meat products, smoked preserved foods, foods subjected to drying by additives such as malt in the production of beer and whiskey, pickled and salty preserved foods[2]. Available data suggest that nitrosamines are found more frequently and at higher concentration in Asian foods than in Western foods[4]. On the other hand, nitrosamines are formed endogenously from nitrate and nitrite. Although the levels have reduced during the last 20 years, sodium nitrites are still widely used as food preservatives in cured meat products. Nitrite is also formed in the human body from oral reduction of salivary nitrate. Vegetables and water are the main sources of nitrate intake. Nitrites are transformed into nitric oxide by gastric acid-catalysed formation, which acts as an nitrosating agent of amines and amides, as consequence of NOC[2]. Under chronic inflammatory conditions, such as precancerous conditions of gastric cancer (GC) and oesophageal cancer (OC), nitrosating agents are overproduced[1]. Studies in volunteers have shown that red meat intake has a consistent dose response in the endogenous formation of NOC measured in faecal samples, while white meat intake has no effect[5].
So far, there is no conclusive epidemiological evi-dence that nitrosamines are carcinogenic to humans, although they produce a wide range of tumours in more than 40 animal species tested[6]. Two important nitrosamines, namely N-nitrosodiethylamine (NDEA) and N-nitrosodimethylamine (NDMA), are classified as probably carcinogenic to humans (group 2A) by International Agency for Research on Cancer (IARC)[7]. One previous comprehensive review on nutrition and cancer[8] concluded that there is convincing evidence that the consumption of the Chinese salted-dry fish is causally associated with the risk of nasopharyngeal cancer with their nitrosamine content being the most plausible agent. Evidence of an increasing cancer risk due to N-nitrosamine and cured meat intake is considered insufficient for GC and possible for OC[8]. A previous review of dietary nitrates, nitrites and NOC and risk of nasopharynx, oesophagus, stomach, pancreas, colorectal and brain cancer concluded that epidemiological evidence related to GC and other tumours remains inconclusive, although the strongest evidence pointed to an increased risk of nasopharyngeal and oesophageal cancer in subjects exposed to high dietary NOC levels[9].
Several foods, such as processed meat and dried salted fish, which are sources of nitrites and/or nitrosamines, are also important sources of salt. Salt produces an inflammatory process leading to damage of the protective stomach mucosa and increases the risk of stomach cancer[8]. H pylori infection may be related to salt and NOC, in enhancing carcinogenesis after the epithelium is damaged[8].
The aim of this article is to review and evaluate the available epidemiological evidence about the association between dietary exposure to preformed nitrosamine and related food intake and gastric and oesophageal cancer risk in humans.
Epidemiological studies (case-control or cohort studies) published between 1985 and 2005 evaluating the relationship between nitrosamines, NDMA, nitrites, food sources of exogenous and endogenous nitrosamines, and oesophageal or gastric cancer risk in males and females were included in the study. Experimental studies were not considered.
We conducted electronic searches in MEDLINE and CANCERLIT databases from 1985-2005. The search strategy included the following terms “oesophageal”, “gastrointestinal”“gastric”, “stomach”, “upper aero digestive tract”, “cancer”, “nitrosamines”, “NOC”, “NDMA”, “processed meat”, “meat”, “intake”, “salted fish”, “dietary patterns”, “nitrites” and “ diet”. The search was supplemented with references included in recovered papers that were not identified in the electronic search. References contained in recent reviews of the literature were also consulted[10].
The following information was gathered from the original publications: study data (author, journal, year, country); epidemiologic design including type of study, number of subjects, follow-up (years), number of cases/controls, type of controls; diet including type and quality of dietary assessment method, number and type of food items; results including the most fully adjusted odds ratio or rate ratios and 95% confidence intervals for the highest and lowest categories of compound/food intake used from each included article. Covariates included in the analysis were also evaluated.
Since information about gastric cancer and NOC and their precursors was heterogeneous, sources of exposure were classified into two groups: nitrosamines and nitrites; food sources of endogenous (red meat) and exogenous (processed meat, beer, pickled and dried vegetables, smoked fish or meat, salted and dried fish or meat) nitrosamines. The odds ratio for each study was plotted in the included Figures, using symbols whose size was proportional to the study size.
A total of 75 publications potentially eligible for inclusion in this review, were identified. After a detailed examination, in which some papers with duplicate or inappropriate information were detected and excluded, 63 studies were finally selected[11-71,78-80]. Of these, 52 studies were case-control studies and 11 were cohort studies. Most of them were carried-out in Asia (35%), Europe (30%), and USA (23%).
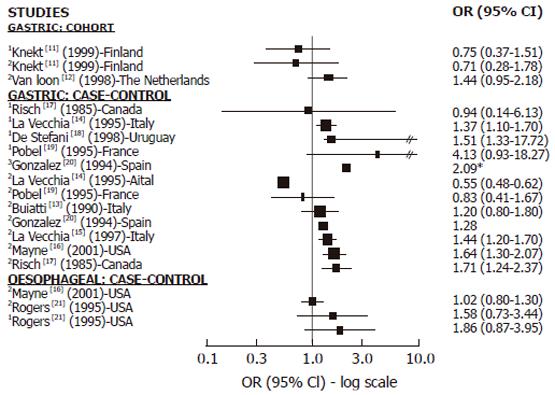
Cohort studies: We found 2 cohort studies[11,12] with information on GC and nitrites, nitrosamines or both (Figure 1). In relation to nitrites, one found a positive but not significant association[12] while the other found no association[11]. Only one cohort study investigated the association between NDMA and GC and found no association[11]. We did not find any cohort studies investigating the relationship between nitrosamine or nitrite intake and OC.
Case-control studies: We found 8 case-control studies with information on GC and nitrites, nitrosamines or both[13-20] (Figure 1). Among the 7 studies on nitrites and GC[13-17,19,20], 5 showed a positive association[13,15-17,20] and 3 achieved statistical significance[15-17]. Two of them[15,16] were large studies, adjusting for all relevant confounding factors. In relation to nitrosamine intake and GC, among the 5 studies published[14,17-20], 4 found a positive association which was statistically significant (SS) in 3 of them[18-20]. We found only 2 case-control studies reporting results in relation to OC which showed no association with nitrite intake[16] or no significantly positive association with nitrite and NDMA intake[21] (Figure 1).
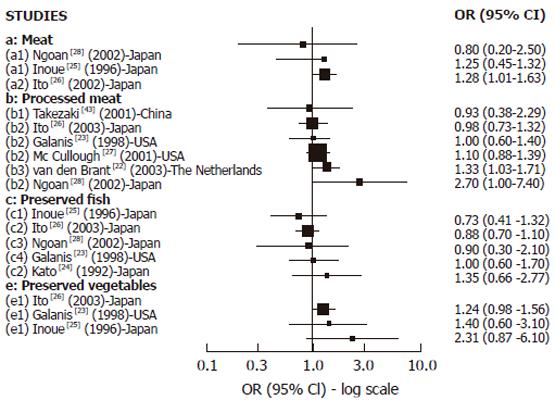
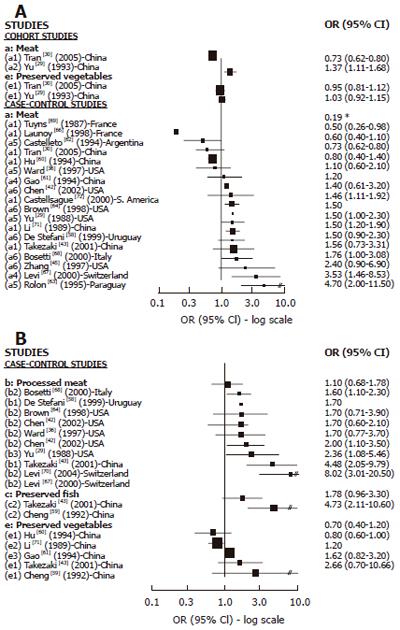
Cohort studies: We found 8 cohort studies with results about GC risk and food sources of exogenous nitrosamines and/or foods than could enhance their endogenous formation[22-28,43] (Figure 2). Only 3 studies reported results in relation to red meat intake. A positive and statistically significant association was observed with pork in one large study[26] while no association was found in the other studies with few GC cases[25,28] . In relation to high processed meat intake, 6 studies reported results[22,23,26-28,43] but the association was positive and SS was found only in 2 studies[22,28] .The largest study[27] did not observe any association, but it was a study based on mortality cases with a relatively small number of food items included in the Food frequency questionnaires (FFQ). Salted, dried or preserved fish intake was associated (but not significantly) with GC risk only in one of the 5 cohort studies reporting results[23-26,28], but in most of them the number of GC cases was too small. For pickled and dried vegetables, 3 studies found positive association but none of them achieved statistical significance[23,25,26]. In the largest study[26] the risk was borderline significant, but in the others the number of cases was too small. In relation to OC, (Figure 4A) we found 2 cohort studies[29,30] reporting results associated with meat intake, which were positive and SS in one study[29], while no association was observed regarding pickled vegetables.
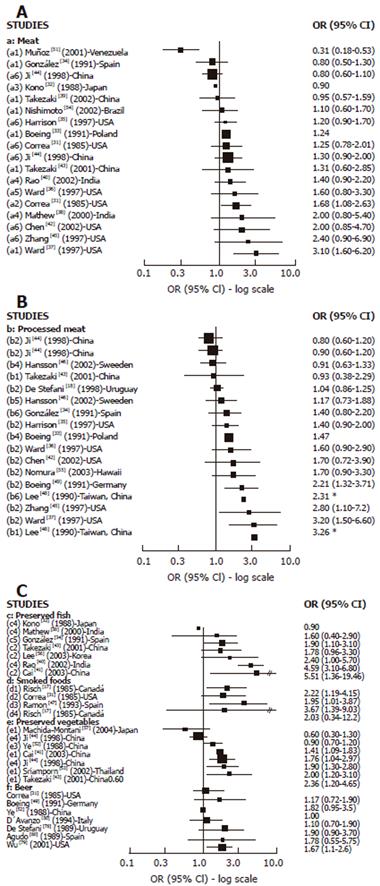
Cases-control studies: We found 16 case-control studies (Figure 3A) reporting results between different types of meat intake (mutton, red meat, beef, fresh meat, grilled meat, pork) and GC risk[31-40,42-45,51,54] and 11[31,33,35-38,40,42-45] of them suggested a positive association with at least one type of meat intake and GC risk, which was statistically significant in 2 studies[31,37] carried out in the USA. We found 14 publications[18,33-37,42-46,48,49,55] with reported results on processed meat intake and GC risk (Figure 3B), and 12 of them[18,33-37,42,45,46,48,49,55] showed a positive association while 4 were statistically significant[37,45,48,49] . For dried/salted or preserved fish (Figure 3C), we found 7 studies[32,34,38,40,41,43,56] reporting results, and 6 described a positive association with GC[34,38,40,41,43,56] while 4 achieved statistical significance[34,40,41,56]. Three studies published results about smoked food intake and GC risk[17,31,65] and all of them showed a positive and significant association. For pickled and preserved vegetables, 5 of 6 studies[41,43,44,52,53,57] showed a positive association with GC, which was statistically significant in all of them[41,43,44,52,53]. Other food sources, such as beer[31,49,50,52,78-80] were analyzed, but only one study showed a significant association with GC[79].
We found 18 case-control studies[29,30,36,42,43,45,58,60-64,68,71,72] which published results on OC and different types of meat intake (Figure 4A). A positive association was observed in 11 of them[29,42,43,45,58,63,64,66,67,70,71] and was SS in 6[29,63,66,67,70,71]. For processed meat (Figure 4B), 8[29,36,42,43,58,64,66,69] of 9 studies[29,36,42,43,58,64,66,67,69] described a positive association with OC, which was SS in 5 studies[29,43,58,67,69]. Only 2 studies[43,59] publishing results on preserved fish intake and OC showed a positive association, being SS in one[59]. The association between preserved vegetable intake and OC was reported in 5 studies[43,59-61,70] and a positive but not significant association was observed in three of them[43,59,61].The two largest studies[61,70] did not find any association.
Although the evidence on nitrite and nitrosamine intake from cohort studies is limited (Table 1), results from case-control studies are quite consistent and support a positive relationship with GC. We did not find any results about the relation with OC from cohort studies and few case-control studies reported results, therefore a conclusion about the relationship between OC and nitrite and nitrosamine intake is impossible.
| Gastric cancer | Oesophageal cancer | |||
| n Studies | n Studies | |||
| (n positive association) | (n positive association) | |||
| [n SS] | [n SS] | |||
| Dietary exposure | Case-control | Cohort | Case-control | Cohort |
| Nitrites | 7 (5) [3] | 2 (1) [0] | 2 (1) [0] | |
| Nitrosamines | 5 (4) [3] | 1 (0) [0] | 1 (1) [0] | |
| Meat | 16 (11) [2] | 3 (2) [1] | 18 (11) [6] | 2 (1) [1] |
| Processed meat | 14 (10) [4] | 6 (2) [2] | 9 (8) [5] | |
| Preserved fish | 7 (6) [4] | 5 (1) [0] | 2 (2) [1] | |
| Preserved vegetables | 6 (5) [5] | 3 (3) [0] | 5 (2) [0] | 2 (0) |
| Smoked foods | 3 (3) [3] | |||
| Beer | 7 (6) [1] | |||
Only few cohort studies have reported results regarding the relationship between red meat intake and GC and OC, showing a positive association in 2 of 3 studies of GC and in 1 of 2 studies on OC. However, there are a large number of case-control studies presenting results on meat intake and GC and OC. Most of them have found a positive association (11 from 16 for GC and 11 from 18 for OC) particularly for OC, and in most of these studies the association is SS. Overall the evidence from case-control studies supports a positive association between meat intake and GC and OC. However, meat is a common substrate to endogenous formation of NOC and also a source of other carcinogenic compounds, such as heterocyclic amines (HA) and polycyclic aromatic hydrocarbons (PAH) which should be taken into account.
Most of the relatively few cohort studies showing results on processed meat intake and GC risk have found no association between them. However, several of these studies considered only a small number of GC cases and food items. No cohort study has shown results on processed meat intake and OC. However a relatively large number of case-control studies have shown quite consistent results supporting a positive association between processed meat intake and GC and OC risk (10 of 14 studies on GC and 8 of 9 studies of OC), which were SS in most of them, particularly regarding OC.
Regarding GC risk and preserved fish intake (particularly dried and salted), inconsistent results were found between case-control and cohort studies. While the case-control studies supported a positive association, the cohort studies did not, although most of them had a small number of cancer cases, and confidence intervals were wide. Therefore, further evidence is needed. No evidence is available from cohort studies in relation with OC and the few case-control studies showed a possible positive association.
Results from case-control studies support a positive association between pickle and other preserved vegetable intake and GC risk. Almost all the studies have shown a positive and significant association. Cohort studies have also observed a positive but not significant association, although the number of cancer cases was small. Most of the studies were carried-out in Asian countries. To date, results on OC risk are inconsistent, but the number of studies is small.
Although the evidence is too limited for a definitive conclusion, it supports a positive association between smoked food intake and GC risk. No evidence exists for OC risk.
So far the evidence is limited, but the majority of studies support a not significantly positive association with GC.
Only 2 cohort[11,12] and 9 case-control studies[16,21,22-28] of 65 studies included in this review have published results on nitrite or nitrosamine intake in relation to GC or OC risk. This could be due to the absence of a complete food composition table for NOC content in foods[72] .For the remainder, the estimation of dietary exposure to NOC and their precursors were done indirectly through foods identified as sources of them.
All the results could be affected by measurement errors in the dietary intake, a common limitation of epidemiological studies. FFQ do not usually collect detailed and complete information about preservation and processing methods of all potential food sources of nitrosamines. In addition, the small number of food items usually included in the FFQ and/or lack of portion size information does not permit accurate estimation of nitrosamine and total food intake. The observed range of total food items in the FFQ varied between 22 and 81 in the studies used. Therefore, it could be expected that not all the studies have achieved an accurate assessment of the intake of these compounds. However, despite the fact that some studies have estimated adequately the exogenous intake of nitrosamines, none of them had information about endogenous NOC. It was reported that endogenous synthesis could contribute to 45%-75% of the total human exposure[3]. However, recent studies carried out in humans have shown that endogenous NOC could be up 30-fold higher than exposure from dietary sources[73-75], suggesting that we are actually measuring a small part of the total dietary human exposure to NOC, and therefore underestimating their effect.
In relation to possible factors that could modify the effect of NOC, few studies considered the intake of vitamin C or smoking habits. None of the studies on GC adjusted their results to consider H pylori infection. This is important because H pylori decreases the levels of vitamin C, a recognized inhibitor of endogenous nitrosamine formation[76]. On the other hand, red meat is a source of iron which is considered an essential growth factor for
H pylori[77]. Therefore, some interaction with meat is expected. Finally, it is also important to take into account interactions with genes, particularly with polymorphisms of metabolic genes involved in the metabolism of NOC or DNA repair genes, which so far have been poorly studied.
At present, available epidemiological evidence from case-control studies on nitrite and nitrosamine intake supports a positive association with GC risk. The evidence in relation with OC is insufficient. There is quite consistent evidence from case-control studies about the positive association between meat and processed meat intake with both GC and OC risk. There is also quite consistent evidence from case-control studies about the positive association between preserved fish and preserved vegetable intake and GC risk, although results are more inconsistent in cohort studies. We have found a suggestive indication of a positive association between GC risk and smoked food intake. However, evidence about the effect of preserved fish and vegetable intake on OC risk is more limited, suggesting that there is no association between beer intake and GC although the evidence is still limited. Overall, more prospective cohort studies are needed to permit definitive conclusions, and should include a large number of cancer cases, dietary questionnaires with a large and detailed number of food items, good estimation of portion size, and control for all known confounding variables in a population with a wide range of food intake.
Evaluating the role of selected genotypes involved in the metabolism of these chemical compounds and DNA repair potentially related to the risk of cancer, is also useful. On the other hand, taking into account that endogenous production seems to be the most important contributor to total NOC exposure, validated methodologies that allow an accurate assessment of production are needed. Therefore, measurement and quantification of DNA adducts of nitrosamines in humans may be the most direct way to assess both sources (exogenous and endogenous) and provide the best biomarker of exposure[6].
In summary, prospective studies with long follow-up periods and validated methodologies quantifying all sources of exposure are needed to confirm the role of NOC in gastric and oesophageal carcinogenesis.
The authors thank Mireia Díaz-Sanchis for her useful collaboration in the Figures.
S- Editor Pan BR L- Editor Wang XL E- Editor Ma WH
| 1. | Bartsch H, Spiegelhalder B. Environmental exposure to N-nitroso compounds (NNOC) and precursors: an overview. Eur J Cancer Prev. 1996;5 Suppl 1:11-17. [PubMed] |
| 2. | Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res. 1991;259:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 337] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Tricker AR. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Hotchkiss JH. Preformed N-nitroso compounds in foods and beverages. Cancer Surv. 1989;8:295-321. [PubMed] |
| 5. | Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, O'Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer. Carcinogenesis. 1996;17:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Shuker DE, Bartsch H. DNA adducts of nitrosamines. IARC Sci Publ. 1994;73-89. [PubMed] |
| 7. | International Agency for Research on Cancer. Overall Evaluation of Carcinogenicity to Humans. IARC monographs. Vol. 1-82. (Last updated January 2004, last accessed March 2004) Available from: http: //monographs.iarc.fr. . |
| 8. | World Cancer Research Fund & America Investigation of Cancer Research, Food, Nutrition and the Prevention of Cancer: a global perspective. Menasha, USA: BANTA Book Group, 1997. . |
| 9. | Eichholzer M, Gutzwiller F. Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutr Rev. 1998;56:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Knekt P, Järvinen R, Dich J, Hakulinen T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer. 1999;80:852-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | van Loon AJ, Botterweck AA, Goldbohm RA, Brants HA, van Klaveren JD, van den Brandt PA. Intake of nitrate and nitrite and the risk of gastric cancer: a prospective cohort study. Br J Cancer. 1998;78:129-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Bonaguri C, Cipriani F, Cocco P, Giacosa A. A case-control study of gastric cancer and diet in Italy: II. Association with nutrients. Int J Cancer. 1990;45:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | La Vecchia C, D'Avanzo B, Airoldi L, Braga C, Decarli A. Nitrosamine intake and gastric cancer risk. Eur J Cancer Prev. 1995;4:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | La Vecchia C, Negri E, Franceschi S, Decarli A. Case-control study on influence of methionine, nitrite, and salt on gastric carcinogenesis in northern Italy. Nutr Cancer. 1997;27:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] |
| 17. | Risch HA, Jain M, Choi NW, Fodor JG, Pfeiffer CJ, Howe GR, Harrison LW, Craib KJ, Miller AB. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol. 1985;122:947-959. [PubMed] |
| 18. | De Stefani E, Boffetta P, Mendilaharsu M, Carzoglio J, Deneo-Pellegrini H. Dietary nitrosamines, heterocyclic amines, and risk of gastric cancer: a case-control study in Uruguay. Nutr Cancer. 1998;30:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Pobel D, Riboli E, Cornée J, Hémon B, Guyader M. Nitrosamine, nitrate and nitrite in relation to gastric cancer: a case-control study in Marseille, France. Eur J Epidemiol. 1995;11:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | González CA, Riboli E, Badosa J, Batiste E, Cardona T, Pita S, Sanz JM, Torrent M, Agudo A. Nutritional factors and gastric cancer in Spain. Am J Epidemiol. 1994;139:466-473. [PubMed] |
| 21. | Rogers MA, Vaughan TL, Davis S, Thomas DB. Consumption of nitrate, nitrite, and nitrosodimethylamine and the risk of upper aerodigestive tract cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:29-36. [PubMed] |
| 22. | van den Brandt PA, Botterweck AA, Goldbohm RA. Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands). Cancer Causes Control. 2003;14:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. Jpn J Cancer Res. 1992;83:568-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Inoue M, Tajima K, Kobayashi S, Suzuki T, Matsuura A, Nakamura T, Shirai M, Nakamura S, Inuzuka K, Tominaga S. Protective factor against progression from atrophic gastritis to gastric cancer--data from a cohort study in Japan. Int J Cancer. 1996;66:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | McCullough ML, Robertson AS, Jacobs EJ, Chao A, Calle EE, Thun MJ. A prospective study of diet and stomach cancer mortality in United States men and women. Cancer Epidemiol Biomarkers Prev. 2001;10:1201-1205. [PubMed] |
| 28. | Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. 2002;87:37-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Yu MC, Garabrant DH, Peters JM, Mack TM. Tobacco, alcohol, diet, occupation, and carcinoma of the esophagus. Cancer Res. 1988;48:3843-3848. [PubMed] |
| 30. | Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 31. | Correa P, Fontham E, Pickle LW, Chen V, Lin YP, Haenszel W. Dietary determinants of gastric cancer in south Louisiana inhabitants. J Natl Cancer Inst. 1985;75:645-654. [PubMed] |
| 32. | Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res. 1988;79:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 187] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Boeing H, Jedrychowski W, Wahrendorf J, Popiela T, Tobiasz-Adamczyk B, Kulig A. Dietary risk factors in intestinal and diffuse types of stomach cancer: a multicenter case-control study in Poland. Cancer Causes Control. 1991;2:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | González CA, Sanz JM, Marcos G, Pita S, Brullet E, Saigi E, Badia A, Riboli E. Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021-1028. [PubMed] |
| 36. | Ward MH, Sinha R, Heineman EF, Rothman N, Markin R, Weisenburger DD, Correa P, Zahm SH. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int J Cancer. 1997;71:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Ward MH, López-Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am J Epidemiol. 1999;149:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev. 2000;9:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Takezaki T, Gao CM, Wu JZ, Li ZY, Wang JD, Ding JH, Liu YT, Hu X, Xu TL, Tajima K. hOGG1 Ser(326)Cys polymorphism and modification by environmental factors of stomach cancer risk in Chinese. Int J Cancer. 2002;99:624-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Rao DN, Ganesh B, Dinshaw KA, Mohandas KM. A case-control study of stomach cancer in Mumbai, India. Int J Cancer. 2002;99:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Cai L, Zheng ZL, Zhang ZF. Risk factors for the gastric cardia cancer: a case-control study in Fujian Province. World J Gastroenterol. 2003;9:214-218. [PubMed] |
| 42. | Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, Russell RM, Weisenburger DD, Tucker KL. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137-144. [PubMed] |
| 43. | Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, Li SP, Su P, Liu TK, Tajima K. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Jpn J Cancer Res. 2001;92:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Ji BT, Chow WH, Yang G, McLaughlin JK, Zheng W, Shu XO, Jin F, Gao RN, Gao YT, Fraumeni JF Jr. Dietary habits and stomach cancer in Shanghai, China. Int J Cancer. 1998;76:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Zhang ZF, Kurtz RC, Yu GP, Sun M, Gargon N, Karpeh M Jr, Fein JS, Harlap S. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer. 1997;27:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Hansson LE, Nyrén O, Bergström R, Wolk A, Lindgren A, Baron J, Adami HO. Diet and risk of gastric cancer. A population-based case-control study in Sweden. Int J Cancer. 1993;55:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Ramón JM, Serra L, Cerdó C, Oromí J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71:1731-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Lee HH, Wu HY, Chuang YC, Chang AS, Chao HH, Chen KY, Chen HK, Lai GM, Huang HH, Chen CJ. Epidemiologic characteristics and multiple risk factors of stomach cancer in Taiwan. Anticancer Res. 1990;10:875-881. [PubMed] |
| 49. | Boeing H, Frentzel-Beyme R, Berger M, Berndt V, Göres W, Körner M, Lohmeier R, Menarcher A, Männl HF, Meinhardt M. Case-control study on stomach cancer in Germany. Int J Cancer. 1991;47:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | D'Avanzo B, La Vecchia C, Franceschi S. Alcohol consumption and the risk of gastric cancer. Nutr Cancer. 1994;22:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Muñoz N, Plummer M, Vivas J, Moreno V, De Sanjosé S, Lopez G, Oliver W. A case-control study of gastric cancer in Venezuela. Int J Cancer. 2001;93:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a casecontrol study in Fujian Province, China. World J Gastroenterol. 1998;4:516-518. [PubMed] |
| 53. | Sriamporn S, Setiawan V, Pisani P, Suwanrungruang K, Sirijaichingkul S, Mairiang P, Parkin DM. Gastric Cancer: the Roles of Diet, Alcohol Drinking, Smoking and Helicobacter pylori in Northeastern Thailand. Asian Pac J Cancer Prev. 2002;3:345-352. [PubMed] |
| 54. | Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, Hanaoka T, Tsugane S. Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in São Paulo. Jpn J Clin Oncol. 2002;32:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control. 2003;14:547-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | De Stefani E, Deneo-Pellegrini H, Mendilaharsu M, Ronco A. Diet and risk of cancer of the upper aerodigestive tract--I. Foods. Oral Oncol. 1999;35:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Cheng KK, Day NE, Duffy SW, Lam TH, Fok M, Wong J. Pickled vegetables in the aetiology of oesophageal cancer in Hong Kong Chinese. Lancet. 1992;339:1314-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Hu J, Nyrén O, Wolk A, Bergström R, Yuen J, Adami HO, Guo L, Li H, Huang G, Xu X. Risk factors for oesophageal cancer in northeast China. Int J Cancer. 1994;57:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Gao YT, McLaughlin JK, Gridley G, Blot WJ, Ji BT, Dai Q, Fraumeni JF Jr. Risk factors for esophageal cancer in Shanghai, China. II. Role of diet and nutrients. Int J Cancer. 1994;58:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Castelletto R, Castellsague X, Muñoz N, Iscovich J, Chopita N, Jmelnitsky A. Alcohol, tobacco, diet, mate drinking, and esophageal cancer in Argentina. Cancer Epidemiol Biomarkers Prev. 1994;3:557-564. [PubMed] |
| 63. | Rolón PA, Castellsagué X, Benz M, Muñoz N. Hot and cold mate drinking and esophageal cancer in Paraguay. Cancer Epidemiol Biomarkers Prev. 1995;4:595-605. [PubMed] |
| 64. | Brown LM, Swanson CA, Gridley G, Swanson GM, Silverman DT, Greenberg RS, Hayes RB, Schoenberg JB, Pottern LM, Schwartz AG. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control. 1998;9:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Launoy G, Milan C, Day NE, Pienkowski MP, Gignoux M, Faivre J. Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer. 1998;76:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Levi F, Pasche C, Lucchini F, Bosetti C, Franceschi S, Monnier P, La Vecchia C. Food groups and oesophageal cancer risk in Vaud, Switzerland. Eur J Cancer Prev. 2000;9:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Tuyns AJ, Riboli E, Doornbos G, Péquignot G. Diet and esophageal cancer in Calvados (France). Nutr Cancer. 1987;9:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 110] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C. Processed meat and the risk of selected digestive tract and laryngeal neoplasms in Switzerland. Ann Oncol. 2004;15:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Li JY, Ershow AG, Chen ZJ, Wacholder S, Li GY, Guo W, Li B, Blot WJ. A case-control study of cancer of the esophagus and gastric cardia in Linxian. Int J Cancer. 1989;43:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 72. | Jakszyn P, Agudo A, Ibáñez R, García-Closas R, Pera G, Amiano P, González CA. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J Nutr. 2004;134:2011-2014. [PubMed] |
| 73. | Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358-2360. [PubMed] |
| 74. | Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132:3522S-3525S. [PubMed] |
| 75. | Hughes R, Cross AJ, Pollock JR, Bingham S. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | Mirvish SS. Blocking the formation of N-nitroso compounds with ascorbic acid in vitro and in vivo. Ann N Y Acad Sci. 1975;258:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 75] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis. 2003;35:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | De Stefani E, Boffetta P, Carzoglio J, Mendilaharsu S, Deneo-Pellegrini H. Tobacco smoking and alcohol drinking as risk factors for stomach cancer: a case-control study in Uruguay. Cancer Causes Control. 1998;9:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control. 2001;12:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Agudo A, González CA, Marcos G, Sanz M, Saigi E, Verge J, Boleda M, Ortego J. Consumption of alcohol, coffee, and tobacco, and gastric cancer in Spain. Cancer Causes Control. 1992;3:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |