INTRODUCTION
Pernicious anemia is characterized by type A (autoimmune) atrophic gastritis, megaloblastic anemia, and neurological and psychiatric findings. It is the most common cause of cobalamin deficiency due to a loss of acid-secreting parietal cells in the gastric body and fundus[1]. It is known that patients with pernicious anemia have a higher risk to develop gastrointestinal malignancies such as gastric adenocarcinoma and carcinoid tumors, or oesophageal squamous cell carcinoma[1-4].
Gastric carcinoid tumors, which arise from hyperplasia of enterochromaffin-like (ECL) cells in the gastric mucosa, comprise 1%-3% of gastric neoplasms[5-7]. Type I carcinoid tumors are associated with ECL cell hyperplasia, hypergastrinemia, achlorhydria, and chronic atrophic gastritis, with or without pernicious anemia[8,9]. Type I carcinoid tumors are detected approximately in 1%-7% of the patients with pernicious anemia[1,3,9,10]. Most of the gastric carcinoid tumors are multifocal, solitary tumors are rare[3,11-14].
This report describes the treatment of a solitary gastric carcinoid tumor by endoscopic polypectomy in a patient with pernicious anemia.
CASE REPORT
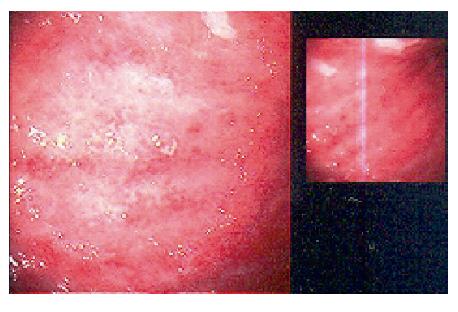
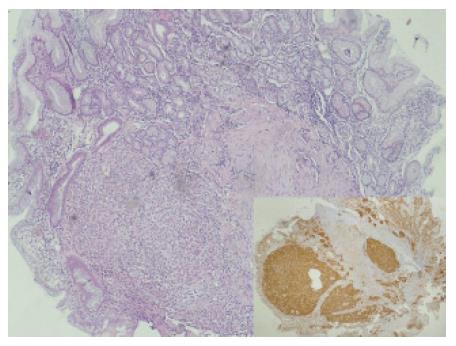
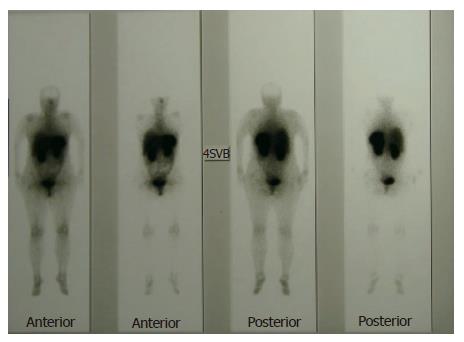
A 48-year-old female patient who was suffering from fatigue for three months was admitted to the hospital. She had no gastrointestinal symptoms, and she negated alcohol abuse. Physical examination revealed pallor, jaundice, and atrophic glossitis. A grade-2 systolic ejection murmur was auscultated on the pulmonary area. Laboratory findings were a hemoglobin level of 59 g/L, a white blood cell count of 2400/mm3, a platelet count of 76 000/mm3, a mean erythrocyte volume of 99 fL, and a reticulocyte count of 2.5%. Peripheral blood smear examination revealed 42% neutrophils, 40% lymphocytes, 12% monocytes, 6% eosinophils, and 3% normoblasts. Moreover, macrocytosis, aniso-poikilocytosis, Howell-Jolly bodies, Cabot ring in erythrocytes, and hypersegmentation in neutrophils were observed. Bone marrow aspiration revealed erythroid hyperplasia (E/M ratio: 6/1), evident megaloblastic changes, and nucleo-cytoplasmic dissociation in normoblasts (Figure 1). While iron parameters were normal, the levels of vitamin B12 and homocsyteine were 62 ng/L (N: 200-900 ng/L) and 91 μmol/L (N: 0-12 μmol/L), respectively. Anti-parietal cell antibody was positive. The biochemical values were normal except those for lactate dehydrogenase 1635 IU/L, total bilirubin 44 mg/L, and indirect bilirubin 34 mg/L. Thyroid function tests were normal, too. By performing upper gastrointestinal tract endoscopy, atrophic gastritis in fundus and corpus and a polypoid lesion in the corpus of 3-4 mm in size were seen (Figure 2). H pylori were not detected with rapid urease test. Endoscopic polypectomy was performed. By histopathological examination, a carcinoid tumor was diagnosed, mainly on the basis of positive stainings for chromogranin A and synaptophysin. H pylori was negative (Figure 3). The levels of fasting serum gastrin and 24 h urinary 5-hydroxyindoleacetic acid were 2000 ng/L and 12.6 μmol (N: 10.4-31.2 μmol), respectively. Spiral computed tomographies of thorax and abdomen were normal. Indium-111 octreotide scan revealed a normal distribution of tracer activity (Figure 4). Electrocardiography, transthoracic Doppler echocardiography, chest X-ray, and gastric endoscopic ultrasonography were normal. Endoscopic re-examination of the stomach was performed and multiple biopsies were obtained from the polypectomy site. The second pathological examination failed to detect any remnant of the carcinoid tumor or neuroendocrine cell hyperplasia. Treatment with vitamin B12 at a dose of 1000 μg/d orally was started[15]. After 6 mo follow-up, the patient was well, and her whole blood counts and the vitamin B12 level were normal.
Figure 1 Megaloblastic changes in the bone marrow.
Figure 2 Polypoid appearance and atrophic gastritis at endoscopy.
Figure 3 Carcinoid tumor positive stained with hematoxylin-eosin A and synaptophysin (x 40).
Figure 4 Indium-111 octreotide scan of the patient.
DISCUSSION
Type I gastric carcinoid tumors result from hypergastrinemia in patients with pernicious anemia. Hypergastrinemia is the result of achlorhydria and atrophic gastritis. Hypergastrinemia acts as a trophic factor for ECL cells, resulting in hyperplasia in gastric mucosa[6,9-11,16,17]. Serum gastrin levels usually range from 740-4000 ng/L in those patients[17]. Although most of those patients are asymptomatic, dyspepsia, pain, nausea, unexplained weight loss, gastrointestinal bleeding, or anemia may be seen[11,13,17]. These carcinoid tumors are usually localized in the gastric fundus or corpus and are less than 2 cm in diameter. Often, they are multifocal. Nodal and hepatic metastasis occur in 2% of the patients[3,11,12,14,17]. Solitary gastric carcinoid tumors that like our patient were reported infrequently[11,13,14,17]. Type II gastric carcinoid tumors, a very rare type, occurs in patients with Zollinger-Ellison syndrome. Most of carcinoid tumors are associated with multiple endocrine neoplasia (MEN) type I. Hypergastrinemia and ECL cell hyperplasia are seen in type II gastric carcinoid tumors. They are multifocal, and their sizes are between 1-2 cm. Local lymph node metastasis can produce[5,6,11]. Type III gastric carcinoid tumors are sporadic, they are not associated with hypergastrinemia, and, in contrast to types I and II, they are invasive large solitary tumors. Carcinoid tumors larger than 3 cm cause metastasis in 66% of the patients while metastasis is detected in only 10% of patients with single tumors smaller than 1 cm[11,17,18].
In our patient, pernicious anemia was diagnosed because of macrocytic anemia, megaloblastic changes in the bone marrow, a decreased vitamin B12 level, an increased homocsyteine level, and atrophic gastritis. The pathological examination of the polyp revealed a solitary gastric carcinoid tumor. Serum gastrin level was high, and urinary 5-hydroxyindoleacetic acid level was normal. There were not symptoms, signs, or laboratory findings of a carcinoid syndrome in our patient. Because of hypergastrinemia in the absence of a Zollinger-Ellison syndrome or MEN type I, the final diagnosis was type I gastric carcinoid tumor.
The best approach modality to the patients with type I gastric carcinoid tumors is endoscopic resection especially in tumors smaller than 1 cm[17,19]. Antrectomy/total gastrectomy is recommended in cases with multiple tumors larger than 1 cm, and with abdominal symptoms such as unexplained weight loss, or aggravation of anemia[6,17,19,20].
We performed endoscopic polypectomy and re-evaluated the patient endoscopically with repeated biopsies. Metastasis was not detected in computed tomographies, pathological examinations, and octreotide scan. Thus, antrectomy/total gastrectomy was not performed. Annual follow-up with endoscopy and computed tomography are planned for the patient. In pernicious anemia, the incidence of gastric carcinoid tumors is increasing as a result of the careful endoscopic and pathological examinations. Type I solitary gastric carcinoid tumors are infrequently detected solitary and endoscopic polypectomy may cure the patient if the tumor is smaller than 1 cm. A follow-up program should be scheduled for patients who underwent polypectomy for any metastasis.