Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4246
Revised: January 20, 2006
Accepted: January 24, 2006
Published online: July 14, 2006
AIM: To investigate genetic instability of gene BRCA1 at locus D17S855, and their relationship with clinicopathological characteristics of gastric cancer in Chinese population.
METHODS: Microsatellite instability (MSI) and loss of heterozygosity (LOH) of gene BRCA1 at locus D17S855 were compared between 37 samples of gastric cancer and corresponding non-cancerous gastric tissue.
RESULTS: MSI at locus D17S855 was positive in 7 of 37 samples of gastric cancer (18.95%). MSI had a close relationship with TNM staging but no relation with lymph node metastasis, histological type or tumor differentiation. MSI positive frequency in TNM I + II (31.58%, 6/19) was much higher than that in TNM III + IV (5.56%, 1/18), (P < 0.05). LOH positive rate was 18.92% (7/37). LOH had no relationship to histological type, tumor differentiation or lymph node metastasis, but LOH positive rate in TNM III + IV was 33.33% (6/18),much higher than that in TNM I + II ( 5.26%, 1/19), (P < 0.05). BRCA1 protein was expressed in 14 of 37 samples of gastric cancer. The positive rates of BRCA1 protein in TNM I + II and TNM III + IV were 57.89% and 16.67%, respectively, (P < 0.05). The positive rate of BRCA1 protein was 77.78% in high differentiation samples, 30.77% in middle differentiation and 12.50% in lower differentiation samples, (P < 0.05).
CONCLUSION: MSI of BRCA1 gene could be used as a molecular marker in early phases of sporadic gastric cancer in Chinese population. LOH occurs at later period of gastric cancer, therefore, it could be used as prognostic factor.
- Citation: Chen XR, Zhang WZ, Lin XQ, Wang JW. Genetic instability of BRCA1 gene at locus D17S855 is related to clinicopathological behaviors of gastric cancer from Chinese population. World J Gastroenterol 2006; 12(26): 4246-4249
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4246
Gastric cancer is one of the most common cancers worldwide, and growing evidence suggests that accumulation of multiple alterations such as activation of proto-oncogenes and inactivation of tumor suppressor genes is responsible for the development and progression of gastric cancer. Genetic instability of oncogenes such as microsatellite instability (MSI) and loss of heterozygosity (LOH) is probably associated with mutations in genes responsible for tumor-genesis, and they play important roles in tumor clinical pathology[1-4]. The studies of MSI and LOH of gastric cancer have been focused on genetic instability of P53, P16 and FHIT, but studies of gene BRCA1 gene are very few. The gene BRCA1 is located in the chromosomal region 17q21. Many studies have reported that BRCA1 is a tumor suppressor gene of breast and ovarian tumors, but only few studies have been done on Chinese gastric cancer[5-7]. The present study was undertaken to investigate MSI and LOH of gene BRCA1 at locus D17S855 in Chinese gastric cancer, their influence on the expression of BRCA1 protein, and their relationship with clinical pathological characteristics of gastric cancer.
A total of 37 formalin-fixed, paraffin-embedded tumor samples and corresponding non-tumor samples from the same sporadic gastric patients were collected. These samples included 30 cases of tubular adenocarcinoma and 7 cases of mucoid adenocarcinoma. All the samples were sectioned into 10 μm sections, and tumor samples contained at least 90% tumor cells. Genomic DNAs from tumor and normal components were extracted by proteinase K and phenol-chloroform extraction methods.
Primers of D17S855 locus were: 5’-GGA TGG CCT TTT AGA AAG TGG- 3’ and 5’-ACA CAG ACT TGT CCT ACT GCC -3’ (Bioasia Shanghai)[8]. Polymerase chain reaction (PCR) was carried out in 50 μL reaction mixtures containing 1.0 mmol/L MgCl2, 10 × Buffer 5 μL, 200 μmol/L dNTP, Taq polymerase 2units and primers 50 pmol/L. PCR was carried out for one cycle of 94°C for 4 min followed by 35 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min, followed by a final extension for 10 min at 72°C. The PCR productions were detected by 2% agarose gel containing ethidium bromide, underwent electrophoresis for 30 min at 15 V/cm, and were examined under ultraviolet light.
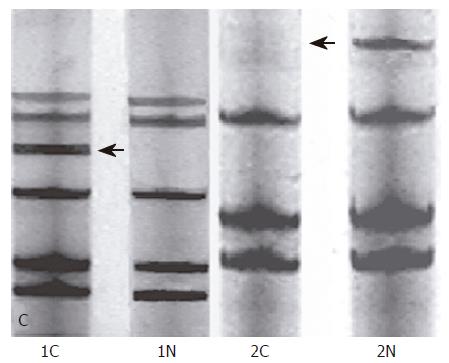
The PCR production and an equal volume of denaturing stop solution (98% deionised formamide, 0.05% bromophenol blue and 0.05% xylene cyanol) were heated for 10 min and then were rapidly cooled on ice. Electrophoresis was carried out on 8% polyacrylamide gels at 100 V for 4 h. After electrophoresis, the gels were ordinarily silver-stained. To detect MSI and LOH, the PCR productions of normal and tumor DNA from the same patients were run in adjacent lanes. MSI was positive as either tumor samples added an allele band or moved as compared with normal tissue. LOH was positive as tumor samples lacked an allele band as compared with normal tissue.
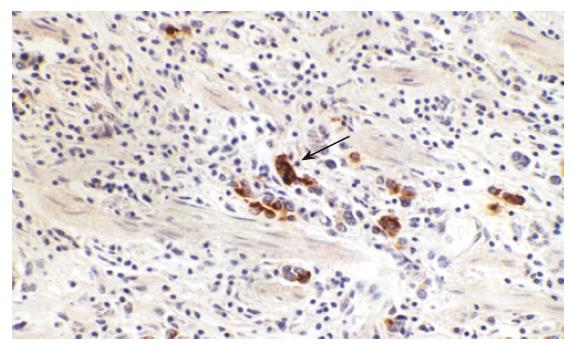
Immunohistochemistry for BRCA1 protein was performed on 5 μm sections from paraffin-embedded tumor tissue which was dewaxed in xylene and dehydrated in a graded ethanol series. The sections were immersed in 3% hydrogen peroxidase for 10 min to block endogenous peroxidase activity, and then rinsed in water. Thereafter, the sections were heated in a microwave for 5 min at 100°C to retrieve antigen. The sections were incubated with primary antibody BRCA1 (Santa Cruz Biotechnology, USA), then the sections were allowed to react by standard Envision method using secondary antibody (DAKO, Denmark). The binding was visualized by DAB, and then the samples were counterstained with hematoxylin.
After immunohistochemistry staining, sections were analyzed by Leica-Qwin computer imaging techniques. We selected twenty continuous high microscopical views that did not overlap. Then we tested the gray-value of background and named as GA. The gray-value of BRCA1 positive granules was named as Ga and area density of BRCA1 positive cells in the view area was named as AAa. We used Excel function to compute the value of PU (positive unit) which represented expression intensity of BRCA1 protein in gastric cancer cell. The highest gray value was 255 in Leica-Qwin system.
Statistical analysis was performed using analysis of variance (AVONA) and t- test. A P value of < 0.05 was considered significant difference.
PU = (Ga - GA)/[(1 - AAa) × 255] × 100
In our experiment, 37 paired normal/tumor DNAs were successfully amplified by PCR method, and were tested by 8% polyacrylamide gels. MSI was positive as added an allele band in tumor tissue as compared with normal tissue; LOH was positive as lacked an allele band in tumor tissue as compared with normal tissue (Figure 1).
Of locus D17S855 at gene BRCA1, the positive frequency of MSI in 37 cases of gastric cancer was 18.92% (7/37) (Table 1). MSI of BRCA1 gene was significantly correlated with clinical TNM staging and degree of tumor differentiation; however, there was no significant difference between MSI-positive and MSI-negative cases in tumor histological type or lymph node metastasis. In tumor node metastasis (TNM) staging, the positive frequency of MSI in stage TNM I + II (31.58%) was more than that in stage TNM III + IV (5.56, P < 0.05), and the frequency of LOH-positive of 37 cases of gastric cancer was 18.92% (7/37) (Table 2). LOH of BRCA1 gene was significantly correlated with clinical TNM staging; however, there was no significant difference between LOH-positive and LOH-negative cases in tumor histological type, tumor differentiation degree or lymph node metastasis. In tumor node metastasis (TNM) staging, the positive frequency of LOH in stage TNM III + IV (33.33%) was higher than that in stage TNM I + II (5.26%, P < 0.05).
| Clinicopatho- logical parameters | Case (n) | MSI positive rates (%) | LOH positive rates (%) | BRCA1 positive rates (%) | BRCA1 expression (PU Mean±S) |
| Histology | 37 | 7 (18.92%) | 7 (18.92%) | 14 (37.84%) | 33.56 ± 2.23 |
| Tubuladenoma | 30 | 6 (20.00%) | 6 (20.00%) | 12 (40.00%) | 32.87 ± 2.93 |
| High | |||||
| differentiation | 9 | 4 (44.44%) 1 (7.69%) | 1 (11.11%) | 7 (77.78%)a | 34.03 ± 1.79 |
| Middle | |||||
| differentiation | 13 | 1 (12 .50 %) | 1 (7.69%) | 4 (30.77%) | 33.49 ± 2.34 |
| Low | |||||
| differentiation | 8 | 1 (14.29%) | 4 (50.00%) | 1 (12.50%) | 33.58 ± 1.67 |
| Mucoadenoma | 7 | 1 (14.29%) | 2 (28.57%) | 32.87 ± 2.59 | |
| Lymph metastasis | |||||
| No | 17 | 5 (29.41%) | 1 (5.88%) | 9 (52.94%) | 34.75 ± 1.78 |
| Yes | 20 | 2 (10.00%) | 6 (30.00%) | 5 (25.00%) | 33.14 ± 2.14 |
| TNM staging | |||||
| Stage I + II | 19 | 6 (31.58%) | 1 (5.26%) | 11 (57.89%) | 33.88 ± 2.38 |
| Stage III + IV | 18 | 1 (5.56%)a | 6 (33.33%)a | 3 (16.67%)a | 33.23 ± 2.34 |
| Grouping | Cases | BRCA1 proteinpositive rates | BRCA1 expression(PU Mean±S) |
| MSI positive group | 7 | 85.71% (6/7) | 34.05 ± 1.91 |
| MSI negative group | 30 | 26.67% (8/30) a | 32.59 ± 2.23 |
| LOH positive group | 7 | 28.57% (2/7) | 32.87 ± 2.35 |
| LOH negative group | 30 | 40.00% (12/30) | 34.03 ± 2.66 |
The expression of BRCA1 protein in gastric cancer was the brown-yellow granules, mostly located in nucleolus, and cytoplasm and membrane of cells were also stained (Figure 2). The frequency of BRCA1 protein-positive in 37 cases of gastric cancer was 37.84% (14/37) (Table 2). Expression of BRCA1 protein was significantly correlated with clinical TNM staging and tumor differentiation degree; however, expression of BRCA1 protein was not associated with tumor histological type or lymph node metastasis. The positive frequency of BRCA1 protein in TNM I + II (57.89%) was much higher than TNM III + IV stage (16.67%, P < 0.05), and BRCA1 positive-rate in well differentiation cases was higher than poor differentiation cases. The frequency of BRCA1 protein- positive decreased as tumor differentiation went down, 77.78% in high differentiation cases, 30.77% in middle differentiation cases, and 12.50% in low differentiation cases (P < 0.05). There was no difference in BRCA1 protein expression intensity analyzed by computer imaging.
In 1994, the breast-cancer susceptibility gene, BRCA1, was identified by positional cloning; subsequently, this gene has been the subject of intensive research effort. BRCA1, located on chromosome 17q21 and encoding a tumor suppressor gene that functions, in part, as a caretaker gene in preserving chromosomal stability, is composed of 22 coding exons distributed over 100 kb of genomic DNA[3]. This gene encodes 1863 amino acids, and more than 200 different germline mutations associated with cancer susceptibility have been identified. Cell cycle checkpoints play an essential role in cell survival by preventing the propagation of DNA damage through cell cycle progression before DNA repair. Recent studies have demonstrated that both ATM and BRCA1 are required for effective S-phase and G2/M-phase checkpoints. Other work has indicated that BRCA1 regulates G2/M DNA damage induced checkpoints through its ability to activate Chk1 kinase and thereby induce signaling cascades downstream of Chk1. BRCA1 functions as a co-activator of p53-mediated gene transcription. Other studies have shown that overexpression of BRCA1 results in the transcriptional activation of GADD45 in a p53-dependent manner. As GADD45 has been implicated in G2/M checkpoints, BRCA1 may in part activate G2/M checkpoints by induction of GADD45 protein[3,9,10].
The association of the BRCA1 gene with susceptibility to breast and ovarian cancer has been strongly demonstrated, and locus D17S855 was found as one of the best candidate loci to detect tumor suppressor genes[10]. Mori et al found that BRCA1 might play an important role in the development of esophagus cancer with high frequent LOH[9]. Therefore, locus D17S855 was used in this experiment to detect MSI and LOH in gastric cancer of Chinese population.
In this study, the frequency of MSI-positive in 37 cases of gastric cancer was 18.92%. MSI of BRCA1 gene was significantly correlated with clinical TNM staging and tumor differentiation degree; however, there was no significant difference between MSI-positive and MSI-negative cases in tumor histological type or lymph node metastasis. TNM staging represents the prognosis of tumor, and we found in the experiment that the frequency of MSI in stage TNM I+ II was more than that in stage TNM III + IV. Therefore, as far as gene BRCA1 is concerned, high frequency of MSI endowed with a good prognosis. Furthermore, the positive frequency of MSI decreased as tumor differentiation went down, tumor differentiation correlated with the development and malignant degree of tumor. According to the experiment results, MSI of BRCA1 was an early period molecule marker of sporadic gastric cancer. Candusso et al found that LOH often occurred in the later stage of cancer, mostly with lymph metastasis[10]. The positive frequency of LOH in 37 cases of gastric cancer in our study was 18.92%, and the positive frequency of LOH in stage TNM III + IV was higher than that in stage TNM I + II. The results suggested that LOH of BRCA1 gene might occur in the later stage of gastric cancer and could be a marker that predicts prognosis of gastric cancer.
The positive frequency of BRCA1 protein in MSI positive group was higher than MSI negative group, but there was no significant difference between LOH positive and negative groups. Expression of BRCA1 protein was significantly correlated with clinical TNM staging and tumor differentiation degree; however, expression of BRCA1 protein was not associated with tumor histological type or lymph node metastasis. The frequency of BRCA1 protein-positive decreased as tumor differentiation went down. All these data suggested that BRCA1 gene might restrain gastric cancer from developing further.
In conclusion, MSI of BRCA1 is an early event in development of sporadic gastric cancer in Chinese population and LOH of BRCA1 gene occurs in later stage, therefore BRCA1 gene might be a suppressor gene for gastric cancer.
S- Editor Wang J L- Editor Zhao JB E- Editor Ma WH
| 1. | Jackson AL, Loeb LA. Microsatellite instability induced by hydrogen peroxide in Escherichia coli. Mutat Res. 2000;447:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Sprecher CJ, Puers C, Lins AM, Schumm JW. General approach to analysis of polymorphic short tandem repeat loci. Biotechniques. 1996;20:266-276. [PubMed] |
| 3. | Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 465] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 4. | Rouba A, Kaisi N, Al-Chaty E, Badin R, Pals G, Young C, Worsham MJ. Patterns of allelic loss at the BRCA1 locus in Arabic women with breast cancer. Int J Mol Med. 2000;6:565-569. [PubMed] |
| 5. | Barnetson R, Jass J, Tse R, Eckstein R, Robinson B, Schnitzler M. Mutations associated with microsatellite unstable colorectal carcinomas exhibit widespread intratumoral heterogeneity. Genes Chromosomes Cancer. 2000;29:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Huiping C, Kristjansdottir S, Bergthorsson JT, Jonasson JG, Magnusson J, Egilsson V, Ingvarsson S. High frequency of LOH, MSI and abnormal expression of FHIT in gastric cancer. Eur J Cancer. 2002;38:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Berney CR, Fisher RJ, Yang J, Russell PJ, Crowe PJ. Genomic alterations (LOH, MI) on chromosome 17q21-23 and prognosis of sporadic colorectal cancer. Int J Cancer. 2000;89:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Garcia-Patiño E, Gomendio B, Lleonart M, Silva JM, Garcia JM, Provencio M, Cubedo R, España P, Ramón y Cajal S, Bonilla F. Loss of heterozygosity in the region including the BRCA1 gene on 17q in colon cancer. Cancer Genet Cytogenet. 1998;104:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Mori T, Aoki T, Matsubara T, Iida F, Du X, Nishihira T, Mori S, Nakamura Y. Frequent loss of heterozygosity in the region including BRCA1 on chromosome 17q in squamous cell carcinomas of the esophagus. Cancer Res. 1994;54:1638-1640. [PubMed] |
| 10. | Candusso ME, Luinetti O, Villani L, Alberizzi P, Klersy C, Fiocca R, Ranzani GN, Solcia E. Loss of heterozygosity at 18q21 region in gastric cancer involves a number of cancer-related genes and correlates with stage and histology, but lacks independent prognostic value. J Pathol. 2002;197:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |