Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4232
Revised: February 10, 2006
Accepted: February 18, 2006
Published online: July 14, 2006
AIM: To investigate the effect of Tetrandrine (Tet) on LPS-induced NF-κB activation and cell injury in pancreatic acinar cells and to explore the mechanism of Tetrandrine preventing LPS-induced acinar cell injury.
METHODS: Male rat pancreatic acinar cells were isolated by collagenase digestion, then exposed to LPS (10 mg/L), Tet (50 μmol/L, 100 μmol/L) or normal media. At different time point (30 min, 1 h, 4 h, 10 h) after treatment with the agents, cell viability was determined by MTT, the product and nuclear translocation of subunit p65 of NF-κB was visualized by immunofluorescence staining and nuclear protein was extracted to perform EMSA which was used to assay the NF-κB binding activity.
RESULTS: LPS induced cell damage directly in a time dependent manner and Tet attenuated LPS-induced cell damage (50 μmol/L, P < 0.05; 100 μmol/L, P < 0.01). NF-κB p65 immunofluorescence staining in cytoplasm increased and began showing its nuclear translocation within 30 min and the peak was shown at 1 h of LPS 10 mg/L treatment. NF-κB DNA binding activity showed the same alteration pattern as p65 immunofluorescence staining. In Tet group, the immunofluorescence staining in cytoplasm and nuclear translocation of NF-κB were inhibited significantly.
CONCLUSION: NF-κB activation is an important early event that may contribute to inflammatory responses and cell injury in pancreatic acinar cells. Tet possesses the protective effect on LPS-induced acinar cell injury by inhibiting NF-κB activation.
- Citation: Zhang H, Li YY, Wu XZ. Effect of Tetrandrine on LPS-induced NF-κB activation in isolated pancreatic acinar cells of rat. World J Gastroenterol 2006; 12(26): 4232-4236
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4232
Many animal and clinical studies have shown that once the progress of acute pancreatitis is initiated, common inflammatory responses are involved. There is a local inflammatory reaction at the site of injury; if marked, this leads to a systematic inflammatory response syndrome (SIRS), and this systemic response is ultimately responsible for the majority of the mortality[1,2]. Lipopolysaccharides (LPS) have been found in the plasma of patients suffering from severe pancreatitis at an early stage of the disease and inflammatory changes resembling acute pancreatitis were described after administration of LPS to several animal species[3,4].
The transcription factor nuclear factor-κB (NF-κB) is a key regulator of cytokine induction. NF-κB represents a family of proteins sharing the Rel homology domain, which bind to DNA as homo- or heterodimers, and activate a multitude of cellular early and stress-related response genes, such as the genes for cytokines, adhesion molecules[5]. NF-κB exists in an inactive form in the cytoplasm of most cells where it binds to an inhibiting protein, IκB. Many stimuli activate NF-κB, including cytokines, LPS and oxidative stress. Stimuli trigger the translocation of NF-κB from cytosol to nucleus where NF-κB binds to its consensus sequence on the promoter-enhancer region of different genes and regulates transcription of specific genes[6]. Recently, some experimental results suggested that the cholecystokinin (CCK) analogue cerulein induced the rapid activation of NF-κB both in vivo and in cultured acinar cells in vitro and activation of NF-κB preceded pancreatic injury and inflammation[7]. Early blockage of NF-κB activation improved the survival of rats with taurocholate pancreatitis[8].
In our previous study[9,10], we have found that LPS could directly induce the calcium overload, NF-κB activation and cell injury in isolated rat pancreatic acinar cell. Furthermore, NF-κB activation could be attenuated by EGTA, a calcium chelater. Based on these results, we speculate that calcium disorder might take part in the NF-κB activation in isolated rat pancreatic acinar cell and calcium channel blocker (CCB) may be useful for the treatment of acute pancreatitis by influencing NF-κB activation in pancreatic acinar cells.
Tetrandrine (Tet), is a main component of fourstamen stephania root which belongs to traditional Chinese drug and one of natural non-specific CCBs. Tet possesses complicated pharmacological effects and has been used in the therapy of many kinds of disease, such as hypertension, arrhythmia, hepatic fibrosis, etc[11]. In former in vivo experiments[12,13], we have observed that Tet could improve the pathological alteration of pancreas and lung and decrease the mortality of rats with acute taurocholate pancreatitis. In an attempt to further explore the mechanism of Tet in the treatment of AP, we adopted isolated pancreatic acinar cells and examined whether Tet possesses the protective effect on LPS-induced acinar cell injury by inhibiting NF-κB activation.
Male Sprague-Dawley rats (200-250 g) obtained from Experimental Animal Center of Chinese Academy of Sciences (Grade SPF II Certificate No. SYXK 2002-0023) were fasted for 12 h. Escherichia coli LPS (WE coli 055:B5) and MTT were purchased from Sigma Co, USA. Tetrandrine was provided by Pharmacology Department of 2nd Medical University of PLA. NF-κB gel shift assay system kits were provided by Promega Co, USA. [γ-32P] ATP was purchased from Shanghai Isotope Company. Antibody against NF-κB p65 was obtained from Santa Cruz Biotechnology Co, CA. Fluorescein isothiocyanate (FITC)-conjugated goat-anti-rabbit antibody was purchased from KPL Company, USA. All other chemicals were supplied from local source at the highest purity available. Hepes buffer salt solution (HBSS) (in mmol/L): NaCl 118, KCl 4.7, CaCl2 2.5, MgCl2 1.13, NaH2PO4· 2H2O 1.0, D-glucose 5.5, HEPES 10, bovine serum albumin 2 g/L, minimum essence medium 2%, L-glutamine 2.0, soybean trypsin inhibitor 0.1 g/L, pH adjusted to 7.4 with NaOH 4 mmol/L.
Pancreatic acinar cells were isolated from male SD rats by collagenase digestion[14]. In brief, after the rat was anesthetized, the pancreas was quickly removed and parenchyma was minced into small fragments and incubated in 10-mL standard buffer containing collagenase V (90 kU/L) at 37°C, and the pancreatic fragments were digested again by collagenase under a shaking condition for 20 min in an incubator. After collagenase digestion, tissue was gently pipetted. Dispersed acini were filtered through a 150-μm nylon mesh, centrifuged 3 times each for 3 min at 100 × g, resuspended with culture media (in HBSS, replacing bovine serum albumin 2 g/L with 10% heat-activated bovine serum) and incubated with 95% O2 , and 5% CO2.
Pancreatic acinar cells were planted in 24 and 96 well plates at 37°C in a CO2 (50 mL/L) incubator and cultured for 4 h, then exposed to different content of media (10 mg/L LPS, 50 μmol/L Tet and 100 μmol/L Tet or culture media as control ) for 30 min, 1 h, 4 h and 10 h respectively.
An MTT assay was employed to assess the viable cell number quantitatively. Briefly, 100 μL of cell suspension (1 × 104 cells) was seeded into 96-well tissue-culture plates. Cells were treated with LPS (10 mg/L), or normal media. Cells in Tet group were treated with 50 μmol/L or 100 μmol/L Tet for 15 min before stimulated by LPS. After treatment with these agents for indicated period, 10 μL MTT (terminal concentration 0.5 g/L) was added into each well, and incubated for 4 h. The formazan crystals were produced by viable cells and dissolved by Me2SO, and the optical density (OD) of the solution was measured at 490 nm of wavelength. Cell viability was directly proportional to OD value. The viable cell number was expressed as a percentage relative to control cells, measured as 100% ×OD490, treated/OD490, control (at 0 h timepoint).
The pancreatic acinar cells that were seeded onto 24-well plates and treated as described above respectively for indicated period were used for immunofluorescence staining. First, the glass slips were pretreated with 0.1% poly-lysine and cell suspensions were incubated at room temperature for 60 min. The cells were then fixed in freshly prepared 4% phosphate-buffered paraformaldehyde for 20 min at 4°C. Then they underwent permeabilization with the addition of phosphate-buffered 0.1% Triton X-100 (wt/vol) for 5 min at room temperature and then incubated in PBS containing 1% bovine serum albumin (wt/vol, blocking solution) for 30 min, also at room temperature. Incubation of the cells was done with either rabbit anti-rat NF-κB p65 polyclonal antibodies (1:100 dilution in blocking solution) or with blocking solution alone as negative control for 1 h at room temperature. This was followed by incubation with goat anti-rabbit antibodies (1:100 dilution in blocking solution) conjugated to FITC for 45 min at room temperature. Finally, the slips were mounted and sealed for examination under a confocal microscope.
After treatment with stimuli for the above indicated period, pancreatic acinar cells were collected to extract nuclear protein for further examination of the activity of NF-κB by EMSA.
Nuclear protein extraction: Nuclear protein extracts were prepared according to Steinle et al[6] with the following modifications. Pancreatic acinar cells (4 × 106) were collected, washed twice with cold phosphate-buffered saline (PBS), homogenized in 1.5% citrate, then centrifuged at 4000 g for 15 min at 4°C, at which point the supernatant was removed. Then 0.3 mL of 0.25 mol/L sucrose-citrate was added into the pellet and mixed to create a nucleic suspension, which was pipetted and paved on the surface of 1.2 mL 0.88 mol/L sucrose-citrate in a tube and centrifuged at 2000 g for 10 min. The resulting pellet was washed twice in 0.05 mol/L Tris-HCl (pH 7.5)-0.15 mol/L NaCl and centrifuged at 2000 g for 10 min at 4°C. After removing the supernatant, an equal volume of KMTD (0.3 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L Tris-HCl (pH 8.0) and 1 mmol/L DTT) was mixed into the pellet and incubated for 1 h. During the incubation, the samples were shaken drastically every 15 min. After incubation, the suspension was centrifuged at 15 000 g for 15 min at 4°C. Aliquots of the nuclear protein extracts from supernatant were stored at -70°C. Protein content of the extracts was determined using Lowry method with bovine serum albumin as the standard.
EMSA: Activity of NF-κB was examined by EMSA[15]. The NF-κB oligonucleotide contains DNA binding sites for NF-κB transcription factors. The double-stranded DNA probe sequence is 5’AGT TGA GGG GAC TTT CCC AGG C 3’and antisense 3’TCA ACT CCC CTG AAA GGG TCC G 5’ (the binding site is underlined). The 3.5 pmol of the appropriate consensus oligonucleotide was end-labeled with [γ-32P] ATP using T4 polynucleotide kinase. The 32P-labeled double stranded oligonucleotide was used as a specific probe. For the competition assay, the unrelated oligonucleotide, AP2 consensus oligonucleotide which lackedκB binding site, was used as a non-specific probe. Nuclear proteins extracted from Hela cell were used as positive control. The nuclear extract equivalent to 5 μg protein was incubated with radiolabeled probe in reaction buffer, and then the mixture was subjected to electrophoresis on 7% acrylamide (wt/vol) gel at 250 V in 0.5 × TBE buffer for 2 h. After being dried, the gel was exposed to X ray film at -70°C for 48 h.
Each n refers to the number of separated experiment. The quantitative data were expressed as mean ± SD and compared using unpaired t-test. The significant differences of incidence of nuclear translocation of NF-κB p65 between LPS group and Tet-pretreated group were analyzed by Chi-square test. P < 0.05 was considered significant.
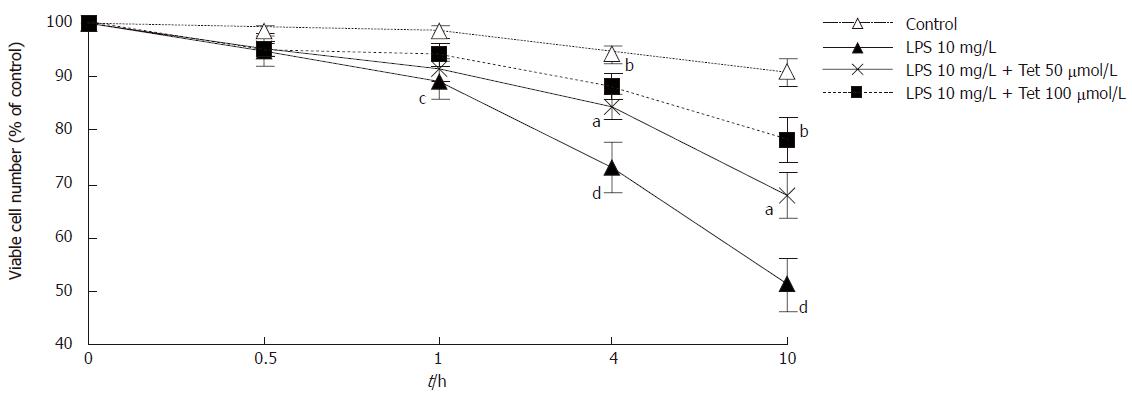
The viable cell number exposed to LPS 10 mg/L decreased with the increase of stimulating time and the difference was significant (P < 0.05 ), compared with control at the same time point. Compared with LPS group, cell mortality in the group pretreated with Tet at 50 μmol/L or 100 μmol/L decreased significantly at each time point (50 μmol/L, P < 0.05, 100 μmol/L, P < 0.01, Figure 1). It suggests that LPS induced cell damage in a time-dependent manner and Tet could attenuate LPS-induced cell damage.
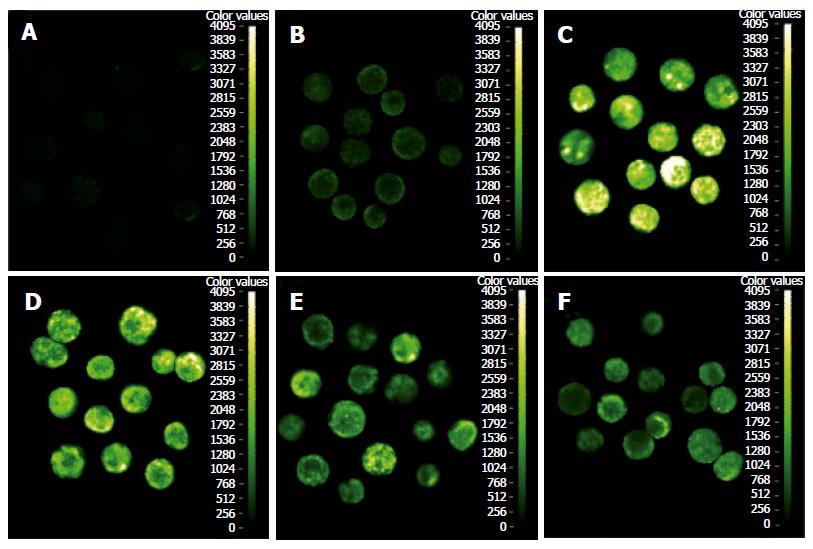
To monitor its activity at a cellular level, NF-κB p65 antibody was used to recognize the product and nuclear translocation signal of the p65. Active p65 level in cytoplasm and its nuclear translocation in pancreatic acinar cells were monitored by immunofluorescence visualization. The cells untreated with LPS showed a slight exclusive cytoplasmic fluorescence pattern (Figure 2B). In contrast, cytoplasmic fluorescence in pancreatic acinar cells treated with LPS 10 mg/L for 1 h and 4 h increased significantly (Figure 2C and 2D). P65 nuclear translocation was seen in > 60% of pancreatic acinar cells incubated with LPS for 30 min and nuclear staining further increased and reached the peak after 1 h of treatment in the LPS group (nuclear translocation ratio > 90%). In the Tet-pretreated group, the pancreatic acinar cells were pretreated with Tet for 15 min and then stimulated with 10 mg/L LPS for 30 min, 1 h and 4 h. Cytoplasmic fluorescence in pancreatic acinar cells was impaired (Figure 2E and 2F) and P65 nuclear translocation was little seen, compared with that in the LPS group (P < 0.05 or P < 0.01, Table 1).
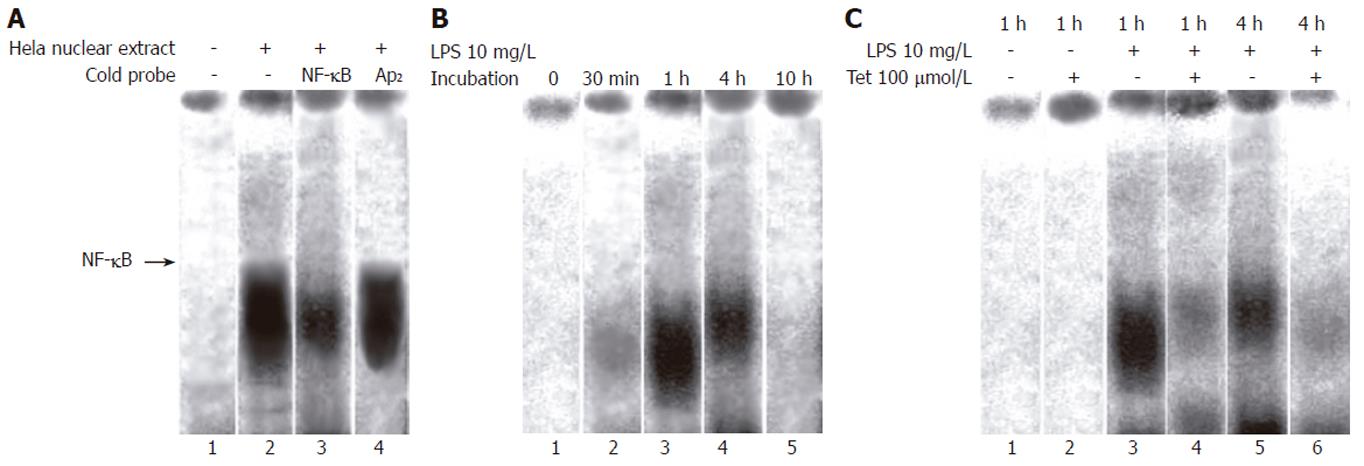
Tet interfered with LPS-induced NF-κB activation: EMSA was performed to detect the binding activity of NF-κB in different group. Pancreatic acinar cells were incubated with LPS or Tet and nuclear extracts were prepared and incubated with a 32P-labeled DNA oligonucleotide containing the recognition site of NF-κB. The specificity of the shift bands was verified by competition assays: The shift bands were suppressed by the incubation with cold NF-κB probe and not influenced by non-specific competition with labeled AP2 probe (Figure 3A). Very little NF-κB binding activity was detected under the unstimulated condition and LPS induced NF-κB binding activity increased in a time dependent manner and NF-κB binding activity was maximal at 1 h after incubation of LPS in 10 mg/L (Figure 3B).
The effect of Tet on LPS-induced NF-κB activation was assessed in pancreatic acinar cells. It was found that in the presence of Tet 100 μmol/L, the optical density and volume of NF-κB shift band markedly decreased than that of LPS group at the same time (Figure 3C).
Lipopolysaccharide (LPS, endotoxin) of the gram negative bacteria outer wall plays a central role in the pathophysiology of the sepsis syndrome[3]. Many experiments demonstrated it is related to the pathogenesis of acute pancreatitis[4,15,16].
LPS induces NF-κB activation in many cells, such as canine tracheal smooth muscle cells, primary rat microglia or monocyte of humans[17-19]. In our previous study[9], we have found that LPS induced the increase of NF-κB binding activity by EMSA and p65 subunit of NF-κB translocation to nuclei by immunofluoresence. However, it is unknown whether there is a more primary factor which precedes the LPS-induced NF-κB activation. Our other study[10] revealed that the first alteration measured after exposing pancreatic acinar cells to LPS system was an increase in the [Ca2+]i which appeared within several hundreds of second. The increase in [Ca2+]i preceded all the other pathological events in the progress of LPS-induced pancreatic acinar cells damage. Intracellular calcium overload exerted an important effect on LPS-induced pancreatic acinar cells damage and egtazic acid, a Ca2+ chelate could attenuate the damage by inhibiting Ca2+ influx. The result suggested that the disorder of calcium homeostasis in pancreatic acinar cells was an important mediator of LPS-induced cell damage. Tando et al observed that caerulein-induced NF-κB/Rel activation requires both Ca2+ and protein kinase C as messenger[5]. We adopted calcium channel blocker (CCB) to observe its effects on the NF-κB activation and viability of isolated rat pancreatic acinar cell.
In our present study, we found that LPS was able to induce the damage in intact pancreatic acinar cells directly at 1 h and the mortality of cell increased with the increase of concentration and stimulating time of LPS. The increase of NF-κB activity preceded the pathological alteration in the progress of LPS-induced pancreatic acinar cells damage. Moreover, a typical CCB, Tet could abrogate LPS-induced NF-κB nuclear translocation, interfere with the DNA binding activity of NF-κB and attenuate cell damage of pancreatic acinar cells. The results show that NF-κB activation is an important early event that may contribute to inflammatory response and cell injury in pancreatic acinar cells and Tet possesses the protective effect on LPS-induced acinar cell injury by inhibiting NF-κB activation. These findings will be helpful to explain the mechanism of Tet in the treatment of AP at the biological level.
We thank Sheng-Nian Wang and Kong-Hua Zhang, from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science for their kind help with the study.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Gloor B, Uhl W, Tcholakov O, Roggo A, Muller CA, Worni M, Büchler MW. Hydrocortisone treatment of early SIRS in acute experimental pancreatitis. Dig Dis Sci. 2001;46:2154-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Raetz CR. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J Bacteriol. 1993;175:5745-5753. [PubMed] |
| 4. | Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Tando Y, Algül H, Wagner M, Weidenbach H, Adler G, Schmid RM. Caerulein-induced NF-kappaB/Rel activation requires both Ca2+ and protein kinase C as messengers. Am J Physiol. 1999;277:G678-G686. [PubMed] |
| 6. | Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Grisham MB. NF-kappaB activation in acute pancreatitis: protective, detrimental, or inconsequential. Gastroenterology. 1999;116:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Li Y, Wang S, Zhang K, Li L, Wu X. LPS-induced NF-kappa B activation requires Ca2+ as a mediator in isolated pancreatic acinar cells of rat. Chin Med J (Engl). 2003;116:1662-1667. [PubMed] |
| 10. | Zhang H, Li YY, Wang SN, Zhang KH, Wu XZ. Effects of lipopolysaccharides on calcium homeostasis in isolated pancreatic acinar cells of rat. Acta Pharmacol Sin. 2003;24:790-795. [PubMed] |
| 11. | Jiang JM, Dai DZ. Research progression of tetrandrine antagonize calcium channel. Zhongguo Yaolixue Tongbao. 1998;14:297-300. |
| 12. | Zhang H, Li YY, Bai JL, Yang YH. Study on therapeutic effect of tetrandrine on acute pancreatitis in rats and its mechanism. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:125-127. |
| 13. | Zhang H, Li YY. Therapeutic effect of tetrandrine on injury of pancreas and lung of rats with experimental acute pancreatitis. Tongji Daxue Xuebao (Yi Xue Ban). 2002;22:363-367. |
| 14. | Kitagawa M, Williams JA, De Lisle RC. Amylase release from streptolysin O-permeabilized pancreatic acini. Am J Physiol. 1990;259:G157-G164. [PubMed] |
| 15. | Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1654] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 16. | Laine VJ, Nyman KM, Peuravuori HJ, Henriksen K, Parvinen M, Nevalainen TJ. Lipopolysaccharide induced apoptosis of rat pancreatic acinar cells. Gut. 1996;38:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yang CM. Lipopolysacchride induction of cyclooxygenase-2 expression is mediated via mitogen-activated protein kinase and nuclear factor-κB pathways in canine tracheal smooth muscle cells. FASEB J. 2002;16:A1147-1152. |
| 18. | Holmes MM, Southerland J, Downey C, Baldwin AS, Offenbacher S. Synnergy of AGEs and P.g. LPS-induced NF-κB activation and Cox-2 expression. FASEB J. 2002;16:A233-239. |
| 19. | Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med. 2000;135:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |