Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4156
Revised: April 15, 2006
Accepted: April 24, 2006
Published online: July 14, 2006
AIM: To investigate the role of IFN-γ inducible protein -10 (IP-10) and regulated upon activation, normal T cell expressed and secreted (RANTES) protein in acute pancreatic allograft rejection in rats.
METHODS: An experimental pancreas transplantation model was established using diabetic SD rats as the recipient, induced by applying streptozocin (STZ). Pancreas transplantation was performed with a physiologic method of portal venous and enteric drainage. Rats were divided into two groups, isograft group (group A, n = 24) and allograft group (group B, n = 24) in which either healthy SD rats or Wistar rats served as donors, respectively. Twelve diabetic or healthy SD rats were used as controls. At d 1, 4, 7, and 10 post transplantation, serum IP-10 and RANTES were assessed by ELISA and their expression in the allografts was determined by immunohistochemistry.
RESULTS: In group B (allograft group), the development of acute rejection was significantly correlated with increased serum concentration and tissue expression of IP-10 and RANTES, with a peak level at d 7 post transplantation. In contrast, there was no obvious change before and after transplantation in group A (isograft group).
CONCLUSION: Our study suggests a possible role of IP-10 and RANTES in acute rejection and early monitoring of chemokines may be helpful in predicting the outcome of pancreas transplantation.
- Citation: Zhu J, Xu ZK, Miao Y, Liu XL, Zhang H. Changes of inducible protein-10 and regulated upon activation, normal T cell expressed and secreted protein in acute rejection of pancreas transplantation in rats. World J Gastroenterol 2006; 12(26): 4156-4160
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4156.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4156
In the past several decades, great success has been made in the transplantation field. However, the acute rejection is still a tremendous obstacle to the development of the transplantation. Acute allograft rejection is a complex process comprising interrelated series of events, such as the recognition of the allograft antigen, the activation and proliferation of the leukocytes, the migration of the leukocytes to the allograft. In the pathogenic process, several kinds of inflammatory molecules are required, such as proinflammatory cytokines, adherence molecules and chemokines. The chemokines are a large family of “chemoattractant cytokines”, including IFN-γ inducible protein-10 (IP-10), regulated upon activation, normal T cell expressed and secreted (RANTES), Mig, etc. They play a critical role in directing leukocytes to the allo-graft and in amplifying intragraft inflammation during rejection[1,2]. In the past years it has become evident that individual proinflammatory chemokines (such as IP-10, RANTES) are indispensable in the rejection process of heart transplantation, kidney transplantation, and lung transplantation, compared to other chemokines[3-5]. However the roles of chemokines in pancreas transplantation are still not well known and pancreas transplantation has its own speciality that differs from other organ transplantations. For example, it is well known that pancreatic grafts are very susceptible to allograft rejection because of the strong immunogenicity of pancreas itself[6]. To assess the changes of chemokines IP-10 and RANTES in the acute rejection of pancreas transplantation, we established the pancreas transplantation model in rats firstly, using a physiologic method of portal venous and enteric drainage[7], and then evaluated the severity of rejection, detected the concentration of serum RANTES and IP-10 using ELISA kits, and examined the expressive position and intensity of IP-10 and RANTES in the allograft pancreas.
The diabetic rats were induced firstly by single intravenous injection of streptozocin (STZ; Sigma, USA) at a dose of 50 mg/kg of body weight, when the closed flock male SD rats weighing 250-280 g were chosen (offered by the Experimental Animal Center of Jiangsu Province, China). After the STZ injection, the nonfasting blood glucose and urine glucose of the rats were assayed before the inducement and measured on alternate day. Only rats with nonfasting blood glucose exceeding 16.8 mmol/L and the strong positive reaction of the urine glucose were selected as recipients. The closed flock male Wistar rats (offered by the Silaike Co. Ltd, Shanghai, China) and healthy SD rats weighing 220-250 g served as donors. The donors and recipients were matched under the condition that the weights of the recipients were more than that of donors by about 30 g.
Rats were divided into two groups, isograft group (group A, n = 24) and allograft group (group B, n = 24), in which either healthy SD rats or Wistar rats served as donors, respectively. Twelve diabetic or healthy SD rats were used as controls.
A physiologic method for pancreas transplantation was adopted, in which the vein was reconstructed by end-to-side anastomosis between the donor portal vein and the recipient superior mesenteric vein, and arterial reconstruction was carried out by end-to-side anastomosis of the donor to the recipient abdominal aorta, and enteric drainage was performed by a side-to-side anastomosis between the duodenum of donors and that of recipients. The level of the recipient’s blood glucose below 11.2 mmol/L at 1 d post operation was regarded as successful transplantation. The recipients were sacrificed at 1, 4, 7, 10 d (n = 6 animals/time point) after transplantation. The 12 rats in control groups were killed at the beginning of the experiment. Blood samples were collected and placed quietly for clotting for 2 h at room temperature before centrifuging for 30 min at 1000 × g, then the serum was pipetted immediately and stored at -70°C. After representative portions of pancreas grafts were removed, some of them were immediately snap-frozen in liquid nitrogen for immunohistology and the rest were fixed in 10% formalin for histopathological examination.
The samples of pancreas grafts were fixed, dehydrated, embedded, sliced, and stained with hematoxylin and eosin following the routine proposal. The classification of acute rejection was stated according to the Nakhleh Classification Criterion[8].
ELISA kits (TPI INC., USA) were used for the determination of serum IP-10 and RANTES, and the procedure was strictly according to the protocol recommended by the manufacturers. The results were expressed as the quantity per mL serum.
For immunohistology, 10 μm frozen sections of pancreas were prepared, fixed in acetone for 10 min, dried in the airy place, and incubated with goat polyclonal IP-10 antibodies and rabbit polyclonal RANTES antibodies respectively. Then, the sections were incubated with rabbit anti-goat IgG and goat anti-rabbit IgG respectively. All the reagents were offered by Santa Cruz Co, USA. The cells stained clearly were regarded as positive ones. According to the percentage of positive cells in the whole infiltrating immune cells, the results of immunohistology were expressed in four grades: negative (the rate of positive cells < 5%), mild positive (the rate of positive cells > 5% and < 25%), moderate positive (the rate of positive cells > 25% and < 50%), strong positive (the rate of positive cells > 50%).
The concentration of serum IP-10 and RANTES were expressed as mean ± SD. The significance of differences was tested using either t-test for means of two samples or analysis of Variance and q-test for means of multiple samples. P < 0.05 was considered as significant.
The acute rejection was classified according to the criterion stated by Nakhleh. In this study, a mild edema appeared around the islet and the acinus 1 d post transplantation both in the allograft and isograft groups. The edema disappeared and no evident rejection was found at 4, 7, and 10 d after the operations in the isograft group, though evident rejection appeared in the allograft group (Table 1).
| Grade I | Grade II | Grade III | Grade IV | |
| 1 d | 5 | 1 | ||
| 4 d | 4 | 2 | ||
| 7 d | 5 | 1 | ||
| 10 d | 6 |
In the allograft group, serum IP-10 concentration was elevated significantly since 4 d and peaked at 7 d after the operations, compared to the control group (P < 0.05). However, no significant difference was found between the isograft group and the control group at the four corresponding phases. The tendency of serum RANTES was similar to that of IP-10, only showing a sharp increase at 1 d after the transplantation in the isograft group (P < 0.05) compared with the control group (Tables 2 and 3).
| 1 d | 4 d | 7 d | 10 d | |
| Isograft group | 40.88 ± 10.76 | 36.74 ± 10.33 | 38.13 ± 12.73 | 31.83 ± 7.55 |
| Allograft group | 43.34 ± 15.29 | 66.26 ± 11.08a | 83.28 ± 16.44a | 70.08 ± 17.65a |
| Control group | 28.76 ± 7.41 | |||
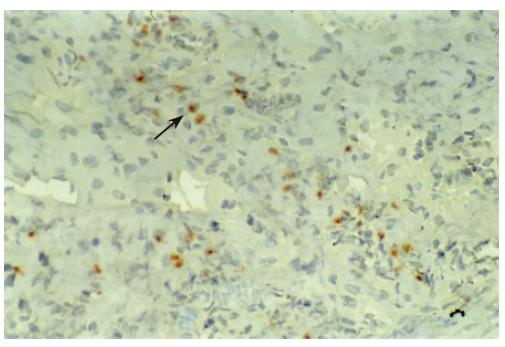
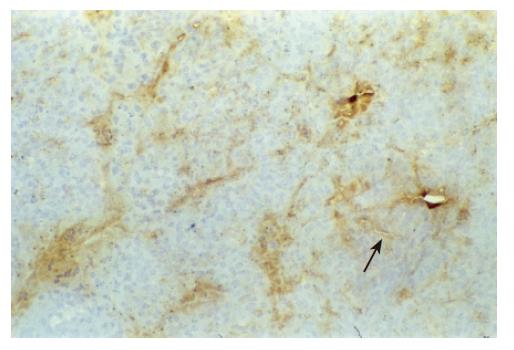
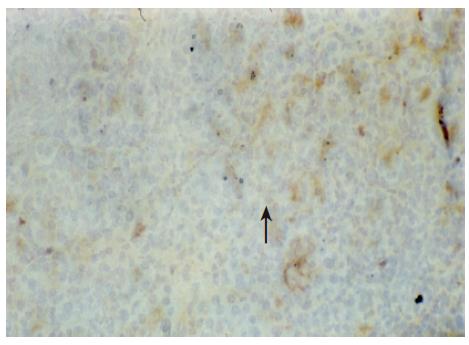
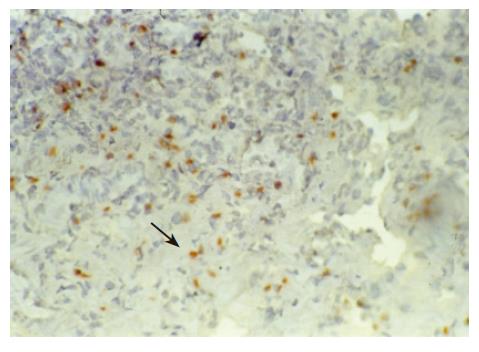
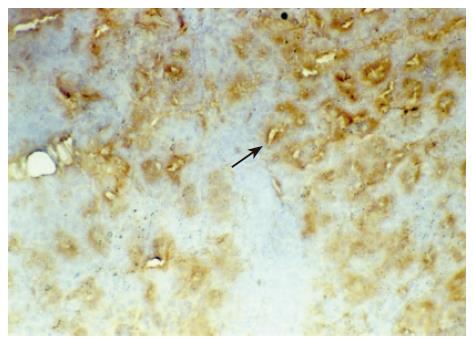
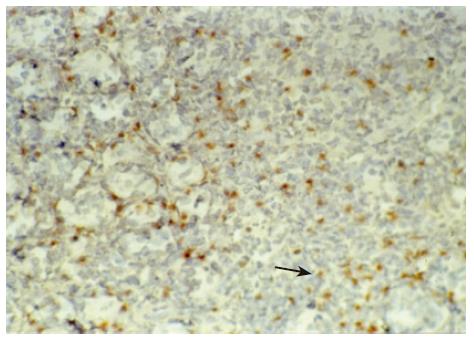
There were no detectable expressions of IP-10 and RANTES protein in the normal pancreas. Mild expression was observed at 1 d after the operation both in the allografts and isografts (Figures 1 and 2). At 4 d after the transplantation, expression of IP-10 and RANTES appeared moderately in the allografts (Figures 3 and 4), whereas their expression could not be detected in the isografts. At 7 d after the operation, the positive expression was strongest in the allografts (Figures 5 and 6). Besides, they were expressed in different places. IP-10 was gathered around the vessels; RANTES was clearly expressed in the lymphocytes in interstitial tissues and concentrated in the narrow space between the inflammatory and normal tissues.
Acute rejection is a very complicated process, in which the cellular and humoral immune mechanisms are involved. In the process of acute rejection, the cellular immune reaction is of primary importance[9]. The circulating lymphocytes firstly adhere to the vessel endothelium, then penetrate the vessel wall, cross the interstitial tissue, migrate into the graft, and finally destroy the graft[10]. In these processes, the chemokines are indispensable. Chemokines accelerate the migration and activation of the leucocytes and lymphocytes through the interaction between the chemokines and their receptors, and play a key role in recruitment of inflammatory cells into an organ transplant[11].
Chemokines are a group of 8-11 kDa proteins, owning four conservative tyrosines in their primary sequence. According to the positional relation between the first tyrosine and the second one, the family are divided into four subfamilies, namely, CXC subfamily, CC subfamily, C subfamily and CX3C subfamily[12].
IP-10 is one of CXC subfamily, mainly coming from activated fibroblasts, monocytes, endothelial cells and T cells[13]. By the interaction between IP-10 and its receptor (CXCR3), IP-10 function in the recruitment of activated Th1 cells specifically and amplify the Th1 reaction. It has been reported that IP-10 and CXCR3 were detected in the allograft in the lung and heart transplantation, and the quantities of IP-10 and CXCR3 are positively related with the severity of rejection response[14]. The latest study performed by Kanmaz et al[15] showed an important correlation between urinary excretion of IP-10 and acute rejection in baboon kidney transplantation and indicated that urinary IP-10 might be a more accurate predictive parameter than serum creatinine to monitor the occurrence of acute rejection of renal transplant.
RANTES belongs to the CC subfamily, produced and secreted by the CD8+ T cells, endothelial cells, and fibroblasts, directing the recruitment of T cells, monocytes, eosinophils to the local tissue[16]. It has been confirmed that RANTES is closely related to the pathogenesis of rejection in heart, lung, kidney transplantation, etc. Mulligan et al[2] found that the protein of RANTES was detectable 6-8 d after the heart transplantation in mice in heterogeneous group. However, the results were opposite in the homogeneous heart transplantation. Sekine et al[17] reported that the mRNA of RANTES was closely positively correlated with the number of infiltrating monocytes, using the models of lung transplantation. Similar results were also obtained in liver transplantation[18]. Some experiments have succeeded in prolonging the functional time of the allografts by inhibiting the interaction between the chemokines and their receptors. Hancock et al[19] reported that the survival phase was elon-gated, provided that the IP-10 knockout mice served as the donors. Yun et al[20] demonstrated that the use of Met-RANTES, the specific antagonist to RANTES, could alleviate the infiltration of CD4+ and CD8+ lymphocytes and attenuate allograft rejection in mice. It seems that IP-10 and RANTES play a pivotal role in the rejection of organ transplantation.
In our experiments, there was no detection of IP-10 and RANTES protein in the normal pancreas. In the isograft group, the proteins of IP-10 and RANTES were detected weakly at 1 d, and restored to the normal level since 4 d post transplantation. In the allograft group, the expression of IP-10 and RANTES protein were also upregulated in the primary phase of acute rejection, and then developed with the severity of the rejection reaction. The trend of serum IP-10 and RANTES was the same as that in the graft. It seems that the detection of IP-10 and RANTES benefits the early diagnosis of acute rejection.
We also noticed that the expressing loci and pattern of IP-10 and RANTES was obviously different. IP-10 was gathered around the vessels; meanwhile, RANTES was clearly expressed in the lymphocytes in the interstitial tissues and crowded in the narrow space between the inflammatory and normal tissues. This result perhaps demonstrated that IP-10 and RANTES play different roles in the acute rejection. IP-10 is a key molecule in directing the lymphocytes to penetrate the endothelium and migrate across the tissue. RANTES, produced by the cells in the inflammatory tissues, can be recognized and bound with its receptors on the surface of passage macrophage, T cells. The activated T cells unregulated the expression of RANTES, and more inflammatory cells were attracted into the graft. The process was cycled and amplified and the inflammation aggravated.
In summary, IP-10 and RANTES play a critical role in the acute rejection after pancreas transplantation. Further research will help the understanding of the pathogenesis of acute rejection. IP-10 and RANTES will be a tool for the early diagnosis and a target for the therapy of acute rejection.
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
| 1. | Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe DM. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Mulligan MS, McDuffie JE, Shanley TP, Guo RF, Vidya Sarma J, Warner RL, Ward PA. Role of RANTES in experimental cardiac allograft rejection. Exp Mol Pathol. 2000;69:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, Feng J, Novick AC, Fairchild RL. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Segerer S, Alpers CE. Chemokines and chemokine receptors in renal pathology. Curr Opin Nephrol Hypertens. 2003;12:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Belperio JA, Strieter RM. Chemokines/chemokine receptors play an important role in the continuum of acute to chronic lung allograft rejection. Curr opinn organ transplant. 2004;9:350-360. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Ito T, Uchikoshi F, Tori M, Miao G, Tanaka S, Maeda A, Akamaru Y, Matsuda H, Nozawa M. Immunological characteristics of pancreas transplantation: review and our experimental experience. Pancreas. 2003;27:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Zhu J, Xu ZK, Miao Y. Improved method for pancreaticoduo-denal transplantation model in rats. Nanjing Yike Daxue Zazhi. 2004;18:308-311. |
| 8. | Nakhleh RE, Sutherland DE, Tzardis P, Schechner R, Gruessner RW. Correlation of rejection of the duodenum with rejection of the pancreas in a pig model of pancreaticoduodenal transplantation. Transplantation. 1993;56:1353-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Zhang AB. Pathogenesis of the acute allograft rejection. Guowai Yixue (Mianyi Fence). 2004;27:4-7. |
| 10. | Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Melter M, Exeni A, Briscoe DM. Chemokines and their receptors in human clinical solid organ transplantation. Curr Opin Organ Transplant. 2002;7:77-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Nelson PJ, Krensky AM. Chemokines, chemokine receptors, and allograft rejection. Immunity. 2001;14:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Inston NG, Cockwell P. The evolving role of chemokines and their receptors in acute allograft rejection. Nephrol Dial Transplant. 2002;17:1374-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Kanmaz T, Feng P, Torrealba J, Kwun J, Fechner JH, Schultz JM, Dong Y, Kim HT, Dar W, Hamawy MM. Surveillance of acute rejection in baboon renal transplantation by elevation of interferon-gamma inducible protein-10 and monokine induced by interferon-gamma in urine. Transplantation. 2004;78:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yun JJ, Fischbein MP, Laks H, Irie Y, Espejo ML, Fishbein MC, Berliner JA, Ardehali A. Rantes production during development of cardiac allograft vasculopathy. Transplantation. 2001;71:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Sekine Y, Yasufuku K, Heidler KM, Cummings OW, Van Rooijen N, Fujisawa T, Brown J, Wilkes DS. Monocyte chemoattractant protein-1 and RANTES are chemotactic for graft infiltrating lymphocytes during acute lung allograft rejection. Am J Respir Cell Mol Biol. 2000;23:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Schenk M, Zipfel A, Schulz C, Becker HD, Viebahn R. RANTES in the postoperative course after liver transplantation. Transpl Int. 2000;13 Suppl 1:S147-S149. [PubMed] |
| 19. | Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Yun JJ, Whiting D, Fischbein MP, Banerji A, Irie Y, Stein D, Fishbein MC, Proudfoot AE, Laks H, Berliner JA. Combined blockade of the chemokine receptors CCR1 and CCR5 attenuates chronic rejection. Circulation. 2004;109:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |