Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4130
Revised: February 10, 2006
Accepted: February 18, 2006
Published online: July 14, 2006
AIM: To investigate whether alphastatin could inhibit human gastric cancer growth and furthermore whether sphingosine kinase (SPK) activity is involved in this process.
METHODS: Using migration assay, MTT assay and Matrigel assay, the effect of alphastatin on vascular endothelial cells (ECs) was evaluated in vitro. SPK and endothelial differentiation gene (EDG)-1, -3, -5 mRNAs were detected by reverse transcription-polymerase chain reaction (RT-PCR). SPK activity assay was used to evaluate the effect of alphastatin on ECs. Matrigel plug assay in nude mice was used to investigate the effect of alphastatin on angiogenesis in vivo. Female nude mice were subcutaneously implanted with human gastric cancer cells (BGC823) for the tumor xenografts studies. Micro vessel density was analyzed in Factor VIII-stained tumor sections by the immunohistochemical SP method.
RESULTS: In vitro, alphastatin inhibited the migration and tube formation of ECs, but had no effect on proliferation of ECs. RT-PCR analysis demonstrated that ECs expressed SPK and EDG-1, -3, -5 mRNAs. In vivo, alphastatin sufficiently suppressed neovascularization of the tumor in the nude mice. Daily administration of alphastatin produced significant tumor growth suppression. Immunohistochemical studies of tumor tissues revealed decreased micro vessel density in alphastatin-treated animals as compared with controls.
CONCLUSION: Downregulating ECs SPK activity may be one of the mechanisms that alphastatin inhibits gastric cancer angiogenesis. Alphastatin might be a useful and relatively nontoxic adjuvant therapy in the treatment of gastric cancer.
- Citation: Chen L, Li T, Li R, Wei B, Peng Z. Alphastatin downregulates vascular endothelial cells sphingosine kinase activity and suppresses tumor growth in nude mice bearing human gastric cancer xenografts. World J Gastroenterol 2006; 12(26): 4130-4136
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4130
Angiogenesis, the development of new blood vessels from pre-existing endothelium is a critical process in many physiological and pathological conditions including embryonic development, organ regeneration, chronic inflammation and solid tumor growth. The process of formation of new blood vessels is complex and involves several discrete steps, such as ECs spreading, migration, proliferation and morphological differentiation of endothelial cells to form tubes. In the last three decades, considerable research has demonstrated that tumor growth and metastasis requires angiogenesis, and micro vascular endothelial cells recruited by the tumor have become an important second target in cancer therapy[1-3]. This is the major reason why angiogenesis has attracted recent attention in the field of pharmacological research. The key for the development of such an angiostatic therapy is to develop useful angiogenesis inhibitors. A number of research groups have shown that various substances are effective in the inhibition of angiogenesis and/or in the treatment of angiogenic diseases like cancer at the experimental animal model level. Various angiostatic factors such as endostatin, angiostatin and thrombospondin have been identified and can block various steps in the tumor angiogenesis pathway. The application of these antiangiogenic agents for cancer treatment is being evaluated through clinical trials and many new angigenesis inhibitors were reported[4-6]. In previous studies, it was reported that alphastatin (24 amino acids) derived from the amino terminus of human fibrinogen could inhibit the growth of murine colonic adenocarcinoma. Significant reduction in tumor volume was found from the fifth day of administration and maintained for 12 d of intraperitoneal injection[7]. S1P, formed through activation of SPK activity, is a bioactive sphingolipid metabolite abundantly stored in platelets. Recently, S1P has been targeted for its potential roles in angiogenesis. It may stimulate DNA synthesis and chemotactic motility of ECs, and also induce tube formation of ECs on Matrigel. Further investigation indicated that S1P predominantly induces angiogenesis via endothelial differentiation gene (EDG), a family of Gi protein-coupled receptor. Activation of EDG receptors triggers several signaling pathways by pertussis toxin (PTX)-sensitive Gi protein. Moreover the signaling pathways activated by S1P have been extensively studied in various cell types. All of these proved that S1P may be a novel mechanism during angiogenesis.
In the present study, we attempt to extend the previous study to observe whether alphastatin inhibits human gastric cancer growth. Importantly, we first report that alphastatin suppress angiogenesis through downregulating ECs SPK activity and reducing ECs S1P level.
Human umbilical vein ECs (HUVECs) and human gastric cancer cells (BGC823) were obtained commercially from ATCC. High glucose Dulbecco’s modified Eagle’s medium (DMEM) and bovine serum albumin (BSA, 1 g/L) were obtained from Sigma chemicals (St. Louis Mo, USA). Growth factor reduced (GFR) Matrigel was purchased from Becton Dickinson (San Jose, Calif., USA). Alphastatin was synthesized by New England Biolabs (Beverly, MA) using standard peptide synthesis techniques and purified to more than 95% using high performance liquid chromatography (HPLC). Hepatocyte growth factor (HGF) was purchased from Peprotech EC Ltd. Transwell plate was purchased from Corning Costar (Cambridge, MA).
HUVECs and BGC823 cells were cultured in DMEM containing 100 mL/L FBS and 10 g/L penicillin-streptomycin in a 50 mL/L CO2 incubator at 37°C. Assessment of ECs migration was performed as recently described with minor modifications[8]. HUVECs were dispersed into homogeneous single cell suspensions after trypsinization. These cells were extensively washed with DMEM containing 1 g/L acid-free BSA and resuspended in the same medium. HUVECs (105) were dispersed onto the upper chamber of Transwell compartment with 8 μm pore size filter. The cells were allowed to adhere for 1 h at 37°C. The medium in the lower chamber was removed and replaced by migration medium containing HGF, HGF plus alphastatin or medium alone. Migration was allowed to proceed for 4 h at 37°C. The remaining cells attached to the upper surface of the filters were carefully removed with cotton swabs. Migrated cells were stained with crystal violet and finally examined by light microscopy. The numbers of migrated cells in at least 10 consecutive fields were evaluated and the average was calculated. Data were expressed as the mean ± SD of the number of migrated cells per field in 5 separate experiments.
HUVECs proliferation was assayed as described previously[9] using standard MTT assay. Alphastatin was dissolved in PBS and diluted in DMEM medium. Cells were incubated in their complete medium containing 100 mL/L FBS, the complete medium plus alphastatin or the starvation medium without FBS. The cells were seeded into 96-well micro titer plates at 5 × 107cells/L in the presence of various concentrations of alphastatin for 24, 48, 72, and 96 h. At each time point, a quarter volume of MTT solution (2 g MTT/L phosphate-buffered saline) was added to each well and each plate was incubated for 4 h at 37°C resulting in an insoluble purple formazan product formation. The medium was aspirated and the precipitates dissolved in 100 μL of dimethyl sulfoxide (DMSO) buffered at PH 10.5. Absorbance at 490 nm was determined using R450 microplate reader (Bio-Rad, USA). Each sample was assayed in five duplicates and repeated at least three times. In order to validate the cytotoxity of alphastatin, the cells were seeded at a density of 5 × 104 cells per well in full growth medium in the absence or presence of alphastatin. After 24 h, the data was collected using CellTiter 96® AQueous One Solution Reagent Kit (Promega, USA).
Endothelial tube formation on Matrigel was conducted as described previously[10]. Matrigel (10 g/L per well) at 4°C was added to a twenty- four-well plates and then allowed to polymerize at 37°C for 1 h. HUVECs (5 × 104cells) were seeded on Matrigel in 1 ml of growth medium and various concentrations of alphastatin. The plates were incubated at 37°C for 18 h. All conditions were triply performed. The formation of tube-like structures by HUVECs was analyzed by fluorescence microscope at × 100 magnification and the total area of tubular structures in five random microscopic fields per well was analyzed using Image tools package 3.0.
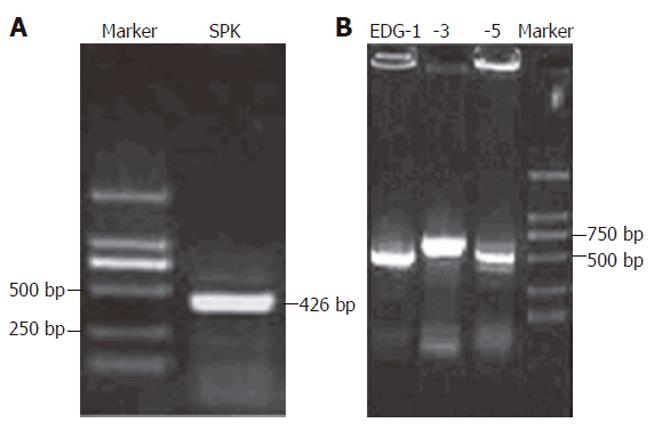
After treated with various dose of alphastatin, total RNA was obtained from HUVECs using the Trizol Reagent kit (Invetrogen, USA). Two μg of total RNA was converted to cDNA by treatment with 100 units of reverse transcriptase and 0.5 μg of oligo-dT primer in 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2 , 0.1 mol/L DTT and 1 mmol/L dNTP at 42°C for 1 h. The reaction was terminated by heating at 70°C for 15 min. Two μL of the cDNA mix was used for enzymatic amplification. Polymerase chain reaction was performed in 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP, 2.5 units of Ex Taq DNA plymerase and 0.1 μmol/L for each of primers such as SPK, EDG-1, EDG-3 and EDG-5. The reaction mixture was repeated for 35 cycles, as heated at 95°C for 45 s, annealed at 57°C for 30 s and extended at 72°C for 60 s. The primers used were 5’ATGCACGAGG TGGTGAACG3’ (sense) and 5’GGAGGCAGGTGTCTTGG AAC3’ (antisense) for the SPK (426 bp); 5’CCGCAAGAACATTTCCAAG (sense) and 5’ACCCACCAACACCCGACAC (antisense) forEDG-1 (608 bp); 5’CCTGC GGGAGCATTA CCA (sense) and 5’CACCTTACGGCTGCTGGAC (antisense) for EDG-3 (637 bp); 5’AAGTTCCACTCGGCAATGTAC (sense) and 5’GCAGC CAGCAGACGA TAAA (antisense) for EDG-5 (556 bp).
After various treatments, HUVECs were washed twice with PBS and harvested by scraping in 0.1 mol/L Tris-HCl buffer (pH 7.4) containing 200 mL/L (v/v) glycerol, 1 mmol/L mercaptoethanol, 1 mmol/L EDTA, 1mmol/L Na3VO4, 15 mmol/L NaF, 10 mg/L leupeptin and aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride and 0.5 mmol/L 4-deoxypyridoxine. Cells were lysed by freezing and thawing. Cytosolic fractions were prepared by centrifugation at 12 000 g for 30 min. One hundred and eighty milliliters of cytosol was incubated with 10 μL of sphingosine (1 mmol/L, dissolved in 50 g/L Triton X-100), 10 μL [γ-32P] ATP (20 mmol/L) containing MgCl2 (200 mmol/L) for 15 min. Sphingosine kinase activity was measured as previously described[11-13].
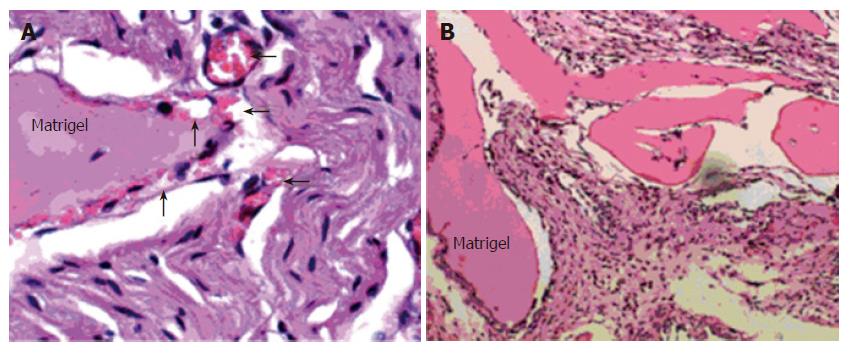
Matrigel plug assay was performed as previously described[14]. Briefly, nude mice were injected subcutaneously with 0.5 mL Matrigel. The injected Matrigel rapidly formed a single solid gel plug. After 7 d, the mice were euthanasia killed and Matrigel plug were removed. It was fixed with 50 g/L formaldehyde phosphate buffer saline and embedded in paraffin. The slides were routinely cut and stained with hematoxylin & eosin (HE). Capillaries were defined as tubular structures containing red blood cells.
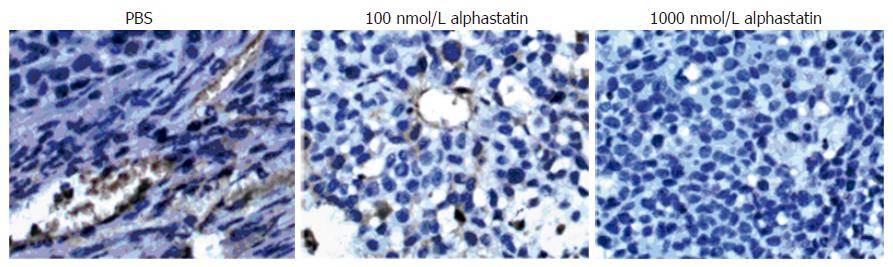
The in-house and governmental animal protection committees approved all of the experiments and the animals were cared according to the guidelines for laboratory animals established by the Chinese government. Female athymic mice (Balb/c, nu/nu, 8 wk of age; weighing about 20 g) were maintained under clean room conditions in sterile rodent micro isolated cages. Animals received sterile rodent chow and water ad libitum. Human gastric cancer cells (BGC 823) were injected subcutaneously (SQ) into the right hind limb (2 × 106 cells in 100 μL of PBS). Tumor cell grew for 8 d until the tumor block established. Animals were randomized for therapy when tumor volume reached 120-160 mm3. Tumor volume was determined three times weekly by direct measurement with calipers and was calculated by the formula “Volume (V) = length × width2× 0.52”. Animals were intraperitoneally daily injected with alphastatin (0.25 mg or 2.5 mg/kg per day in 7 mice respectively) or PBS (control in 7 mice). The observations were terminated for ethical reasons when the tumor volume became large compared with the animal size. Tumor for histological analysis was harvested from three animals at d 12 after the start of antiangiogenic therapy. At the end of observation, tumor tissues were fixed in buffered formalin and were embedded in paraffin. Tissue slices (5 μm) were cut and stained with HE. To assess the tumor angiogenesis, immunohistochemical staining was performed using the anti-mouse Factor-VIII monoclonal antibody. Tumor angiogenesis was quantified by counting the number of positively stained microvessels in 10 randomly chosen fields at × 400 magnifications.
The data were presented as mean ± SD and analyzed by a statistical software of SPSS 10.0 for Windows program using Student’s t-test and the analysis of variance (ANOVA). Significant differences were considered when P < 0.05.
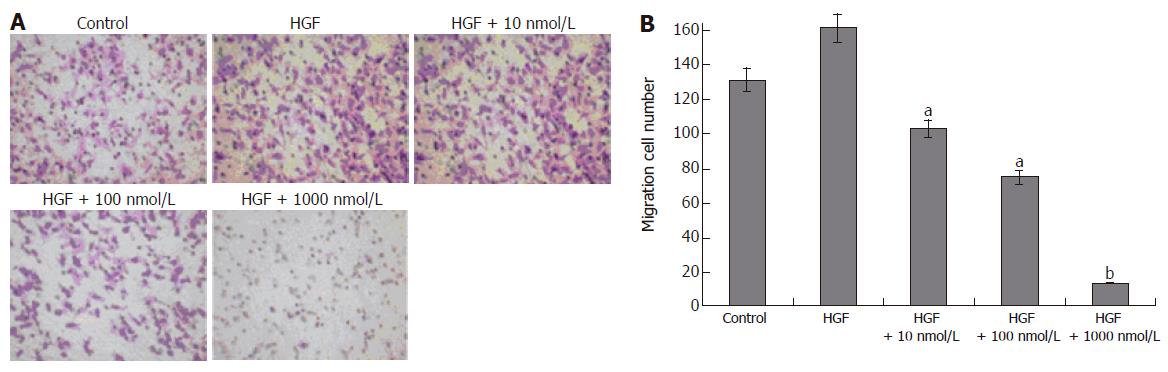
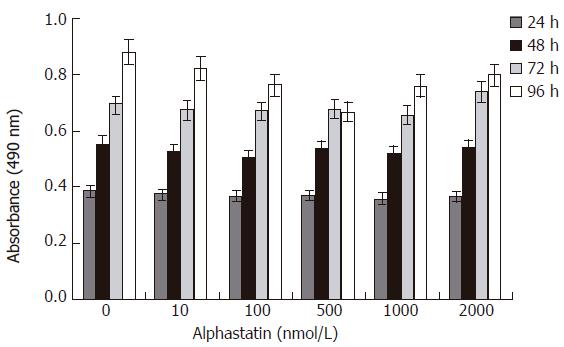
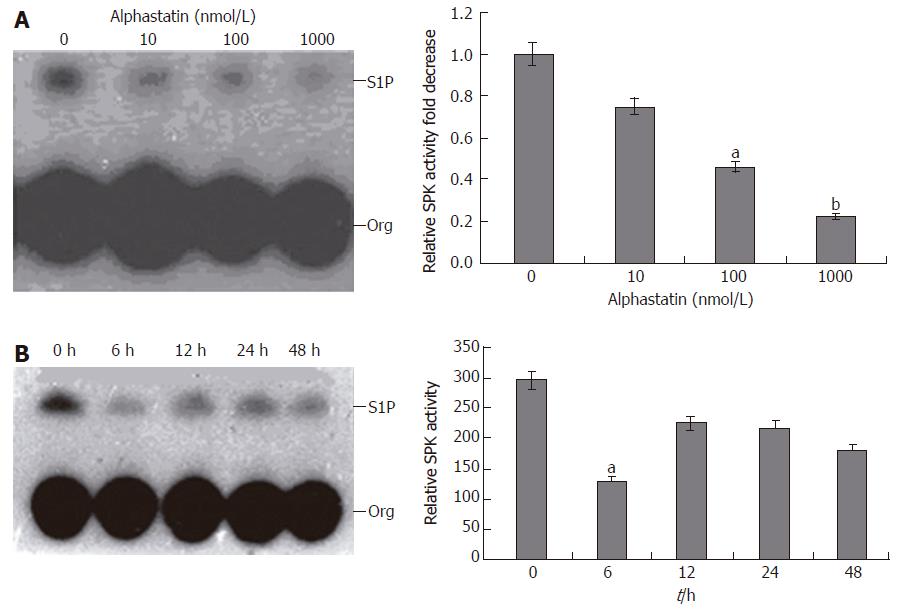
Vascular endothelial cell migration is critical for tumor angiogenesis. To determine the effects of alphastatin on migration of HUVECs induced by chemoattractant media (HGF), we counted the number of cells that had migrated to the bottom of the Transwell membrane. Alphastatin significantly inhibited HUVECs migration in response to HGF in a dose-dependent manner (103 ± 4 and 75 ± 3 vs 131 ± 4, P < 0.05; 13 ± 1 vs 131 ± 4, P < 0.01, Figure 1). Alphastatin had no effect on cell proliferation even up to 2000 nmol/L for 96 h (Figure 2) .The cytotoxicity of alphastatin was assessed and no detectable cytotoxic effect at those doses was found in vitro (data not shown).
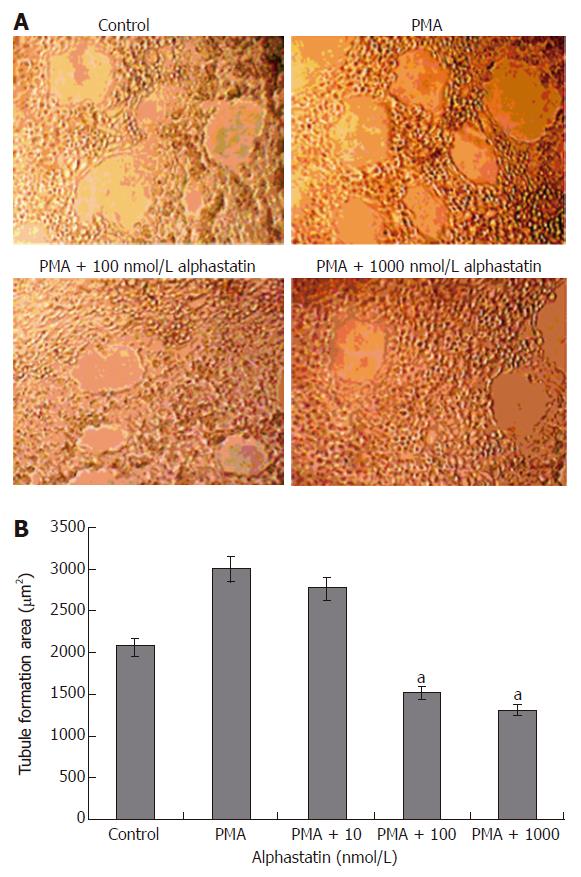
When HUVECs were placed on growth factor-reduced Matrigel surface, they formed a branching and anastomosing network of capillary like tubules with multicentric junctions over 18 h. Alphastatin inhibited the formation of tubular networks induced by PMA (25 μg/L) on Matrigel in a dose-dependent manner (Figure 3). A remarkable inhibition of tube formation was observed in the presence of 100 nmol/L (1508.96 ± 29.89 μm2vs 2150 ± 31.05 μm2, P < 0.05). At the same size field, there was longer distance between tubes and less tubules at alphastatin-treated group. Tube formation was quantitatively estimated by measuring the area covered by the tube network using the image analysis program.
Before measurement of SPK activity, RT-PCR was carried out using specific primers for SPK, EDG-1, EDG-3 and EDG-5 to assure expression of them in HUVECs. It was revealed that SPK, EDG-1, EDG-3 and EDG-5 were expressed in HUVECs (Figure 4). Whether alphastatin could change HUVECs SPK activity is a mistery. HUVECs were starved overnight in serum-free medium before the addition of alphastatin. Then, these cells were stimulated with various concentration of alphastatin for different times. Alphastatin could downregulate SPK in a dose-dependent manner. When HUVECs were treated with alphastatin at a concentration of 100 nmol/L, the cellular SPK activity reached a minimum value at 6 h (Figure 5). These data showed that alphastatin leads to downregulation of SPK through stimulation of HUVECs.
Matrigel was injected into nude mice with or without alphastatin. Reduction in the number of blood vessels was observed at 100 nmol/L alphastatin group (Figure 6). Sections of experimental tumors were stained with antimouse Factor-VIII antibody.
Microvessel density was less in alphastatin-treated group than in PBS group (Figure 7).
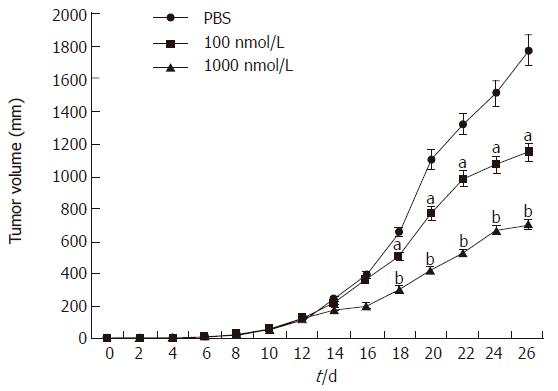
For tumor growth quantification, we used the human BGC823 gastric cancer cell lines. After injecting tumor cells, tumors were allowed to grow until the block established (10 d).Then mice were randomized and divided into control group (PBS: n = 7) and therapy group (alphastatin: n = 7). The tumor in control group steadily grew up to a final tumor volume of 1771 ± 262 mm3 over the same period as the therapy group. However, the growth rate of the tumor in the therapy group was significantly reduced between d 18 and d 26 (P < 0.05 and P < 0.01, Figure 8), and a final tumor volume was only 1145 ± 114 mm3 and 612 ± 173 mm3 respectively. Moreover, alphastatin injection appeared to be well tolerated in vivo and had no significant effect on animal body mass and general condition.
Previous studies show that alphastatin is a potent antiangiogenic agent[7]. However, little is known about whether it has any effect on human derived tumors and what mechanism related to the antiangiogenic activity of alphastatin. In the present study, we therefore tried to solve the question above. Gastric cancer is one of the most common malignancies in Asia. However, so far there is no effective therapeutic measure for this highly malignant disease. Recent studies have demonstrated that angiogenesis is a prerequisite for development and growth of different tumors[15-18] Anti-angiogenic targeting of the neovasculature within tumors is considered one of the most promising strategies in the search for novel antineoplastic therapies[19]. Strategies for anti-angiogenic drugs were described as follows: (1) interference with endothelial cell migration and proliferation; (2) interference with endothelial cell tubule formation and (3) interference with some factors involved in angiogenesis pathway[20-25]. The present study shows that alphastatin inhibits angiogenesis in vitro and in vivo. Furthermore, for the first time we described that alphastatin could suppress human gastric cancer growth in nude mice through downregulating vascular endothelial cell SPK activity. In migration assay, we chose HGF as chemoattractant media because it is a multifunctional factor regulating cell growth, motility and migration for a variety of cell types including endothelial cell, epithelial cell and hepatocyte. Its effect of chemotaxis is greater than vascular endothelial growth factor (VEGF). Duan et al showed that HGF/c-Met activates SPK via ERK1/2 and PI3K pathways. SPK activation plays an important regulatory role in HGF-induced migration of endothelial cells[26]. Lee et al showed that S1P strongly induces the formation of tube network of HUVECs which has the characteristics of strong intercellular interaction and sustained survival of large population of cells on Matrigel[11]. These morphological changes of HUVECs may occur mainly through binding of S1P with EDG-1. In addition, S1P in part and indirectly stimulates HUVECs proliferation through direct secretion of angiogenic factors, because an increase in the expression of VEGF mRNA was detected within 24 h after treatment of HUVECs with S1P[27-29]. In the present study, alphastatin inhibited the migration and tube formation of HUVECs, but no significant effect on cell proliferation even up to 2000 nmol/L. It was in SPK activity assay shown that alphastatin down regulated HUVECs SPK activity and reduced cellular S1P production. From the data above, it could be concluded that alphastatin inhibited the process of angiogenesis through interference with HUVECs SPK activity.
In the in vivo Matrigel plug assay, there was only granuloma formation without significant neovasculature in alphastatin group. Therefore, the antiangiogenic action of alphastatin in vivo may be due to its inhibitory effects on endothelial cells stimulated by VEGF as shown in the present study[5]. In our tumor model, the BGC823 tumors continued to grow over the 12-d injection period, whereas nude mice injected daily with alphastatin demonstrated significant reduction in tumor volume from d 8 of administration and this was maintained for 14 d of injection. The inhibition was in a dose-dependent manner. Immunohistochemical studies of tumor tissues revealed decreased microvessel density in alphastatin-treated animals as compared with control group. It is also likely that alphastatin down regulated SPK activity and reduced endothelial cell S1P production, resulted in inhibition of angiogenesis.
In conclusion, our current studies provide evidence for anti-angiogenic activities of alphastatin. It induced human gastric cancer vascular growth ceasing in vivo, inhibited tube formation on Matrigel and disrupted HUVECs migration. More importantly, the inhibition of angiogenesis was through down regulating HUVECs SPK activity and this may be a novel mechanism that alphastatin exerts antiangiogenic activity. All of these findings hint that, as an new anti-angiogenic agent, alphastatin might be a prototype anti-tumor drug.
We thank Professor Li-Sheng Wang and Professor Hai-Feng Duan of Beijing Institute of Radiation Medicine for their help in this study.
S- Editor Wang J L- Editor Zhao JB E- Editor Bi L
| 1. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6490] [Article Influence: 259.6] [Reference Citation Analysis (0)] |
| 2. | Bamias A, Dimopoulos MA. Angiogenesis in human cancer: implications in cancer therapy. Eur J Intern Med. 2003;14:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 510] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Aoki K, Watanabe K, Sato M, Ikekita M, Hakamatsuka T, Oikawa T. Effects of rhizoxin, a microbial angiogenesis inhibitor, on angiogenic endothelial cell functions. Eur J Pharmacol. 2003;459:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45:143-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 2002;23:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Staton CA, Brown NJ, Rodgers GR, Corke KP, Tazzyman S, Underwood JC, Lewis CE. Alphastatin, a 24-amino acid fragment of human fibrinogen, is a potent new inhibitor of activated endothelial cells in vitro and in vivo. Blood. 2004;103:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. Plasmin-induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J Biol Chem. 2002;277:33564-33570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772-3778. [PubMed] |
| 10. | Guidolin D, Vacca A, Nussdorfer GG, Ribatti D. A new image analysis method based on topological and fractal parameters to evaluate the angiostatic activity of docetaxel by using the Matrigel assay in vitro. Microvasc Res. 2004;67:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, Kim KW, Kwon YG. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 298] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Phongkitkarun S, Kobayashi S, Kan Z, Lee TY, Charnsangavej C. Quantification of angiogenesis by functional computed tomography in a Matrigel model in rats. Acad Radiol. 2004;11:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Nicolella D, Maione P, Gridelli C. Targeted therapies: focus on a new strategy for gastrointestinal tumors. Crit Rev Oncol Hematol. 2003;47:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Tozer GM. Measuring tumour vascular response to antivascular and antiangiogenic drugs. Br J Radiol. 2003;76 Spec No 1:S23-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237-268. [PubMed] |
| 20. | Alessi P, Ebbinghaus C, Neri D. Molecular targeting of angiogenesis. Biochim Biophys Acta. 2004;1654:39-49. [PubMed] |
| 21. | Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144-1155. [PubMed] |
| 22. | Zhang HT, Bicknell R. Therapeutic inhibition of angiogenesis. Mol Biotechnol. 2003;25:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Brack SS, Dinkelborg LM, Neri D. Molecular targeting of angiogenesis for imaging and therapy. Eur J Nucl Med Mol Imaging. 2004;31:1327-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 25. | Bicknell R. The realisation of targeted antitumour therapy. Br J Cancer. 2005;92 Suppl 1:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Duan HF, Wu CT, Lu Y, Wang H, Liu HJ, Zhang QW, Jia XX, Lu ZZ, Wang LS. Sphingosine kinase activation regulates hepatocyte growth factor induced migration of endothelial cells. Exp Cell Res. 2004;298:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758-7768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Ren J, Dong L, Xu CB, Pan BR. Expression of sphingosine kinase gene in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:602-607. [PubMed] |