Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4086
Revised: February 10, 2006
Accepted: February 18, 2006
Published online: July 7, 2006
AIM: To record calcium and potassium currents in acutely isolated smooth muscle cells of mesenteric arterial branches in rats.
METHODS: Smooth muscle cells were freshly isolated by collagenase digest and mechanical trituration with polished pipettes. Patch clamp technique in whole-cell mode was employed to record calcium and potassium currents.
RESULTS: The procedure dissociated smooth muscle cells without impairing the electrophysiological characteristics of the cells. The voltage-gated Ca2+ and potassium currents were successfully recorded using whole-cell patch clamp configuration.
CONCLUSION: The method dissociates smooth muscle cells from rat mesenteric arterial branches. Voltage-gated channel currents can be recorded in this preparation.
- Citation: Cai Q, Zhu ZL, Fan XL. Whole-cell recordings of calcium and potassium currents in acutely isolated smooth muscle cells. World J Gastroenterol 2006; 12(25): 4086-4088
- URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4086
Ca2+and K+ channels, two components that form action potential, are especially important for the regulation of cell excitability because they depolarize and repolarize excitable cells in response to depolarizing events. Voltage-gated K+ channels are implicated in governing cell excitability[1] and setting resting potential[2]. Studies have shown that molecular mechanisms, including protein phosphorylation, gene expression, muscle contraction and neurotransmitter release, are regulated by Ca2+ channels[3]. Patch clamp technique is a powerful tool for studying the electrophysiological properties of biological membranes. Although some methods for patch clamp using freshly isolated smooth muscle cells have been described[4], these methods need multiple enzymes[5] or complex procedure[6]. An improved method was described in the present study for the fast and reliable isolation of smooth muscle cells of mesenteric arterial branches by single collagenase digest and mechanical trituration. The present study was to establish the method of whole-cell recordings of calcium and potassium currents in acutely isolated smooth muscle cells. It can be used to observe the effects of drugs and some gastrointestinal tract disease on ion channels of smooth muscle cells of gastrointestinal tract.
The Krebs-Ringer solution containing 120.7 mmol/L NaCl, 5.9 mmol/L KCl, 15.5 mmol/L NaHCO3, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgCl2, 2.5 mmol/L CaCl2 and 11.5 mmol/L glucose was used in this study with its pH adjusted to 7.4. The Ca2+-free physiological saline solution (PSS) containing 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L HEPES, and 10 mmol/L glucose was also used in the study. The pH was adjusted to 7.4 with Tris-base. For recording Ca2+ currents, the cells were continuously perfused with the extracellular solution containing 140 mmol/L TeaCl, 5 mmol/L BaCl2, 10 mmol/L HEPES, 10 mmol/L glucose. The pH was adjusted to 7.4 with Tris-base. Recording pipettes were filled with the following intracellular solution containing 130 mmol/L CsCl, 10 mmol/L EGTA, 3 mmol/L MgATP, 0.3 mmol/L Na2GTP, 10 mmol/L HEPES, 10 mmol/L glucose. The pH was adjusted to 7.2 with CsOH. For recording potassium currents, the cells were continuously perfused with HEPES-buffered physiological containing 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 10 mmol/L HEPES, 10 mmol/L glucose. The pH was adjusted to 7.4 with Tris-base. The voltage dependent Ca2+ channels were blocked by using 0.2 mmol/L CdCl2. Intracellular solution containing 140 mmol/L KCl, 10 mmol/L EGTA, 3 mmol/L MgATP, 10 mmol/L HEPES, and 10 mmol/L glucose. The pH was adjusted to 7.2 with Tris base. The internal recording solution was filtered through a Millipore 0.2 μm syringe filter before use and frozen till the experiment. Both external and internal solutions were of analytical grade. DTT, TTX, EGTA, MgATP, Na2GTP, HEPES, CsCl, TeaCl, collagenase and bovine serum albumin were obtained from Sigma.
Animals were provided by the Experimental Animal Center of Medical College of Xi’an Jiaotong University. All procedures were carried out in accordance with the United States Public Health Services Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University. The smooth muscle cells were freshly dispersed as described previously[7]. Briefly, male Sprague-Dawley adult rats weighing 120-150 g were anesthetized with ether. The mesenteric arterial branches were dissected. The connective tissue and the endothelium were carefully removed under microscope (XTZ-E, Shanghai Shangguang Microscope Co., Shanghai, China) and the smooth muscle was cut into 1-2 mmol/L sections. The tissues were preincubated with oxygenated Ca2+-free PSS for 40 min at 18-22°C, then incubated in oxygenated Ca2+-free PSS containing 0.3% collagenase I, 0.3% bovine serum albumin,and 6 mg DTT for 25-30 min at 32 °C. The tissues were washed three times with oxygenated Ca2+-free PSS, and then gently agitated with a blunt-tipped glass pipette until the solution became cloudy. The debris was then removed with a fine 200 μm nylon mesh. The cell suspension was stored at 4°C and used within 5 h after cell harvesting.
The smooth muscle cells were transferred to a recording chamber mounted on an inverted microscope (DMIRB, Leica, Germany) for 15 min. Only spindle-shaped cells were selected for experimentation. Electrophysiological recordings were performed with standard whole cell voltage-clamp technique[8]. Recording pipettes were made from borosilicate glass capillaries using a puller (model p-97, Sutter Instrument, Novato, CA) and heat-polished to allow gigaohm seal formation. The resistance of the recording pipettes filled with internal recording solution was 3-6 MΩ in bath solution. The junction potential between the patch pipettes and bath solution was nullified immediately before GΩ seal formation. Cell capacitances were read from the potentiometer to set transient capacitance to zero. This value was used to calculate the cell size. After pipette and cell transient capacitance were compensated with the appropriate controls on the amplifier, the membrane was ruptured with gentle suction to obtain the whole cell voltage-clamp configuration. Series resistance was routinely compensated by 60%-70% and monitored periodically. Voltage-gated Ca2+ currents and delayed rectifier potassium currents (IKD) were recorded as previously described[9]. Electrophysiological recordings were performed at room temperature (22-25°C).
All data were analyzed by pCLAMP CLAMPFIT procedures (Axon Instrument, USA). Current-voltage relations of voltage-gated channels were plotted by Origin 6.0 software (Microcal Software, USA).
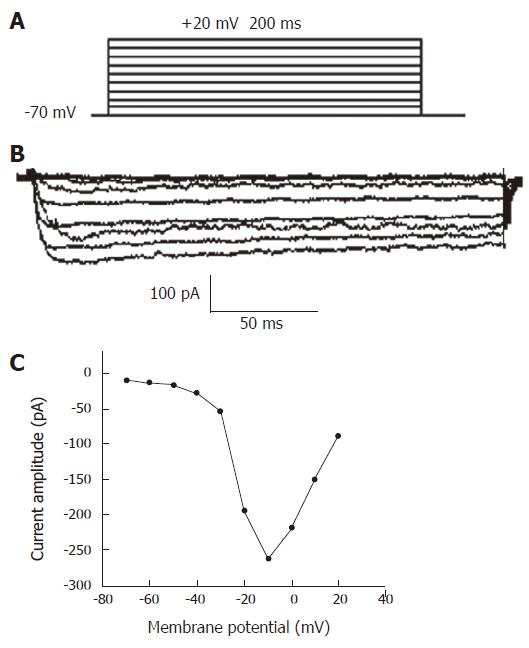
Voltage-gated Ca2+ currents were elicited by 200 ms depolarizing potential from -70 mV to +20 mV with an increment of 10 mV. At 5 s intervals, a holding potential was -70 mV (Figure 1A). The example of raw Ca2+current traces for isolated smooth muscle cell is shown in Figure1B. No significant inward current was observed until the depolarizing step reached -40 mV. Currents reached maximum amplitude at approximately -10 mV in each case, and became small with stronger depolarization. The current amplitude in dependence of the potential could be shown as an I-V curve (Figure1C). The maximal current peak amplitude of the curve was -228.64 pA.
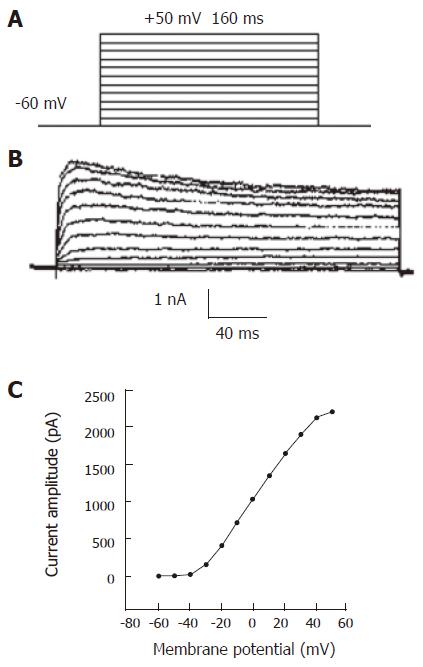
IKD was elicited by 160 ms depolarizing pulses (-60 mV to +50 mV) at 2 s intervals, from a holding potential of -60 mV (Figure 2A). Figure 2B shows a series of raw current traces evoked. The outward currents showed a slow activation and inactivation during depolarizing pulses, and increased with increasing test potentials. The currents were sensitive to TeaCl, a potassium current blocker. The currents recorded under the condition were outward delayed rectifier potassium currents. Current–voltage (I-V) curve of IKD was obtained by plotting the currents evoked against test pulse (Figure 2C). The maximal current peak amplitude of the curve was 2216.75 pA.
Theoretically, isolation with enzyme irreversibly digests the protein-including ion channels in cell membrane, which could not be excluded with our method. Compared with methods reported previously[5,6], the present methods have the following advantages. The isolating process is simple, fast and easy to manipulate using a single enzyme instead of several enzymes. The yield of the freshly isolated smooth muscle cells with high quality is increased markedly, leading to a GΩ seal. The activity and dose of enzyme could influence the cells’ quality and quantity. Excessive enzyme destroys the normal structure of cells and causes swelling of cells. The membranes of the remanent living neurons are easy to break before GΩ seal easily, which is the key to successful patch clamp experiments. On the other hand, the enzyme is not enough to disrupt the connection between cells. When pipetted, the sections are difficult to disperse. Incubation of sections is beneficial to their recovery. Careful and slow trituration of tissue sections is necessary and air bubble should be avoided to prevent cell damage.
In conclusion, Ca2+ and K+ channel currents can be successfully recorded in acutely isolated smooth muscle cells. Our method can dissociate smooth muscle cells from rat mesenteric arterial branches, and is a powerful tool for functional analysis at the level of individual smooth muscle cells.
Instrument for patch clamp was provided by the Key Laboratory of Environment and Genes Related to Diseases (Xi’an Jiaotong University), Ministry of Education.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799-C822. [PubMed] |
| 2. | Parsons RL, Barstow KL, Scornik FS. Spontaneous miniature hyperpolarizations affect threshold for action potential generation in mudpuppy cardiac neurons. J Neurophysiol. 2002;88:1119-1127. [PubMed] |
| 3. | Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 502] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Liu H, Xiong Z, Sperelakis N. Cyclic nucleotides regulate the activity of L-type calcium channels in smooth muscle cells from rat portal vein. J Mol Cell Cardiol. 1997;29:1411-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Yamakage M, Hirshman CA, Namiki A, Croxton TL. Inhibition of voltage-dependent Ca2+ channels of porcine tracheal smooth muscle by the novel Ca2+ channel antagonist RWJ-22108. Gen Pharmacol. 1997;28:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Zheng HF, Li XL, Jin ZY, Sun JB, Li ZL, Xu WX. Effects of unsaturated fatty acids on calcium-activated potassium current in gastric myocytes of guinea pigs. World J Gastroenterol. 2005;11:672-675. [PubMed] |
| 7. | Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol. 2001;423:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12857] [Cited by in RCA: 14048] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 9. | Li XL, Zheng HF, Jin ZY, Yang M, Li ZL, Xu WX. Effect of actin microfilament on potassium current in guinea pig gastric myocytes. World J Gastroenterol. 2004;10:3303-3307. [PubMed] |