Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4009
Revised: March 17, 2006
Accepted: March 27, 2006
Published online: July 7, 2006
AIM: To study the effects of disruption of sarA gene on biofilm formation and antibiotic resistance of Staphylococcus epidermidis (S. epidermidis).
METHODS: In order to disrupt sarA gene, the double-crossover homologous recombination was applied in S. epidermidis RP62A, and tetracycline resistance gene (tet) was used as the selective marker which was amplified by PCR from the pBR322 and inserted into the locus between sarA upstream and downstream, resulting in pBT2△sarA. By electroporation, the plasmid pBT2△sarA was transformed into S. epidermidis. Gene transcription was detected by real-time reverse transcription-PCR (RT-PCR). Determination of biofilm was performed in 96-well flat-bottomed culture plates, and antibiotic resistance was analyzed with test tube culture by spectrophotometry at 570 nm respectively.
RESULTS: A sarA disrupted strain named S. epidermidis RP62A△sarA was constructed, which was completely defective in biofilm formation, while the sarA complement strain RP62A△sarA (pHPS9sarA) restored the biofilm formation phenotype. Additionally, the knockout of sarA resulted in decreased erythromycin and kanamycin resistance of S. epidermidis RP62A. Compared to the original strain, S. epidermidis RP62A△sarA had an increase of the sensitivity to erythromycin at 200-400 μg/mL and kanamycin at 200-800 μg/mL respectively.
CONCLUSION: The knockout of sarA can result in the defect in biofilm formation and the decreased erythromycin and kanamycin resistance in S. epidermidis RP62A.
-
Citation: Tao JH, Fan CS, Gao SE, Wang HJ, Liang GX, Zhang Q. Depression of biofilm formation and antibiotic resistance by
sarA disruption inStaphylococcus epidermidis . World J Gastroenterol 2006; 12(25): 4009-4013 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4009
Staphylococcus epidermidis (S. epidermidis), a normal inhabitant of human skin and mucous membranes, is the predominant cause of foreign-body-associated infections. The pathogenesis of S. epidermidis infections is correlated with its ability to form biofilms on polymer surfaces[1,2]. Biofilm formation proceeds in two phases[3,4]. Primary attachment of bacterial cells to a polymer surface is a complex process influenced by a variety of factors, including hydrophobic interactions, presence of host proteins, and specific bacterial proteins and polysaccharides like the capsular polysaccharide adhesin, the autolysin AtlE, and other staphylococcal surface proteins[5,6]. This is followed by the second phase leading to accumulation of bacteria in a multilayered biofilm embedded in an amorphous glycocalyx. This phase is a multifactorial process that is influenced by a number of factors. Among the most important of these is polysaccharide intercellular adhesion (PIA). Synthesis of polysaccharide intercellular adhesin is essential for bacterial cell accumulation because it mediates cell-to-cell adhesion of proliferating cells[7,8]. PIA consists of two polysaccharide species which are composed of β-1, 6-linked 2-deoxy-2-amino-D-glucopyranosyl residues containing non-N-acetylated amino groups, phosphate, and succinate. The enzymes required for polysaccharide intercellular adhesin synthesis are encoded within the icaADBC operon, mutation of which results in a reduced capacity to form a biofilm in S. epidermidis[7]. SarA, a central regulatory element that controls the production of Staphylococcus aureus (S. aureus) virulence factors, is essential for the synthesis of PIA and the ensuing biofilm development in this species[9,10]. Based on the presence of a sarA homolog, we hypothesized that SarA could also be involved in the regulation of the biofilm formation process in S. epidermidis. To elucidate the possible role of SarA in biofilm formation, we used a genetic approach and constructed an S. epidermidis sarA::tet knockout mutant of the biofilm-forming strain S. epidermidis RP62A. Biofilm formation and ica expression of the mutant were compared with the phenotypes of the corresponding wild-type strain and a complemented strain that carried a sarA copy in an expression vector.
We were interested in the potential role of SarA in the response of S. epidermidis to antimicrobial agents. Therefore, we used this sarA knockout mutant and determined its influence on erythromycin and kanamycin resistances in S. epidermidis RP62A (i.e. ATCC35984).
The bacterial strains and plasmids used in this study are listed in Table 1. Tryptic soy broth (TSB) was used to grow Staphylococcus strains. Luria-Bertani (LB) was used for E coli. Chloramphenicol was used at 15 μg/mL, ampicillin at 100 μg/mL and tetracycline at 10 μg/mL. Bacteria were grown on Congo red agar (CRA) plates, which were composed of TSB agar supplemented with 5% sucrose (A.R.) and 0.8 mg of Congo red/L (A.R.). All DNA manipulations and handling of E coli were performed in accordance with standard protocols[11]. Manipulations with Staphylococcus were performed as described previously[12,13].
| Strains or plasmids | Relevant characteristics | Sources or references |
| Strains | ||
| S. epidermidis ATCC12228 | Biofilm negative | ATCC |
| S. epidermidis RP62A (ATCC 35984) | Biofilm positive Kanr, Eryr, Apr | ATCC |
| S. epidermidis RP62A△sarA | sarA deletion on the chromosome, Tetr, sarA::tet | This study |
| S. epidermidis RP62A (pHPS9sarA) | S. epidermidis RP62A△sarA harboring pHPS9sarA | This study |
| S. aureus RN4220 | Restriction-negative, intermediate host for plasmid transfer from E. coli to S. epidermidis | [14] |
| E. coli DH5α | Φ80dlacZ△M15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK- mK+) supE44 relA1 deoR △(lacZYA-argF) U169 | Promega Co. |
| Plasmids | ||
| pBR322 | Donator of tet gene (Tetr). Apr Tetr | [15] |
| pBluescriptSK | E. coli cloning vector.Apr | [11] |
| pBT2 | Temperature-sensitive shuttle vector. Apr (E. coli) Cmr (Staphylococcus) | [16] |
| pBT△sarA | Integration vector for homologous recombination of the△sarA gene in S. epidermidis; tet inserted into sarA locus as resistance selection marker | This study |
| pHPS9 | Expression shuttle vector | [17] |
| pHPS9sarA | pHPS9 inserted sarA gene | This study |
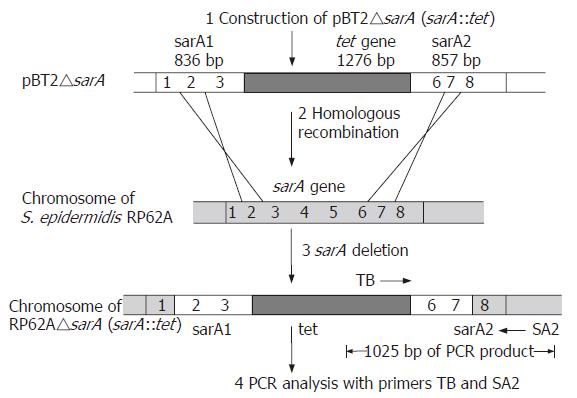
In order to analyze its function in S. epidermidis, sarA was replaced by a tetracycline resistance gene (tet) by homologous recombination. The upstream of 836 bp, fragment 1, was amplified using primer pairs (1) 5’-ACGAAGCTTC TGTAACATCT AGTGACAA-3’ and (2) 5’-ACGCTGCAGT TTAATCTGTC AGCATAAGTG-3’ with HindIII and PstI respectively. The downstream of 857 bp, fragment 2, was amplified using primer pairs (3) 5’-ACGCTGCAGA TTATAAACAA CCTCAAGTTG-3’ and (4) 5’-ACGGAATTCG GGCATCATTG CGAGTGA-3’ with PstI and EcoRI respectively. The two fragments were cloned into the multi-cloning region of temperature-sensitive E. coli-Staphylococcus shuttle vector pBT2[16], resulting in plasmid pBT2-1. A fragment of 1276 bp containing the entire tet gene was amplified by PCR from the pBR322, using the primers (5) 5’-CGCGCGGCCG CTTCTCATGT TTGACAGCTT-3’ and (6) 5’-GCGAGATCTT CAGGTCGAGG TGGCC-3’. The tet gene was inserted into the vector pBT2-1, resulting in plasmid, pBT2△sarA. Following passage through the restriction-negative strain S.aureus RN4220, pBT2△sarA was reisolated and transformed into S. epidermidis RP62A by electroporation. Replacement of the chromosomal S. epidermidis RP62A sarA wild-type gene was obtained by double-crossover integration of the sarA::tet insert of pBT2△sarA following a temperature shift to the nonpermissive temperature (42°C) of the shuttle vector[18]. Tetracycline-resistant and chloramphenicol-sensitive colonies were isolated. The sarA::tet integrations were confirmed by PCR detection (Figure 1 step 4) and the nucleotide sequencing was carried out by Shanghai Bioasia.
RNA purification, real-time reverse transcription-PCR (RT-PCR) and analysis of RT-PCR data were performed as previously described[19], with the following oligonucleotide primer pairs: for gyrB transcript, (7) 5’-TTATGGTGCT GGACAGATAC A-3’ and (8) 5’-CACCGTGAAG ACCGCCAGAT A-3’; for icaA transcript, (9) 5’-AACAAGTTGA AGGCATCTCC-3’ and (10) 5’-GATGCTTGTT TGATTCCCT-3’. The gyrB gene was compositively expressed in S. epidermidis and thus used as an internal standard in these RT-PCR experiments[19].
The biofilm production assay was performed by cultivation of the S. aureus and S. epidermidis strains on CRA plates as described by Freeman et al[14]. The black, rough and dry colonies on CRA plates indicated the biofilm production. In contrast, the biofilm-negative strains formed red, smooth colonies.
Strains were cultivated overnight (16 h) in 96-well flat-bottomed tissue culture plates at 37°C in TSB growth medium. Based on the optical densities (OD570) of the biofilm, the strains were classified as non-adherent strains (OD570≤ 0.120).
Three antibiotics were used to determine the impact of sarA mutant on S. epidermidis resistance to erythromycin or kanamycin. Erythromycin or kanamycin was added into test tubes with a twofold serial dilution scheme (0, 25, 50, 100, 200, 400, 800 μg/mL)[20,21]. The inoculum was derived from overnight liquid cultures in TSB and was inoculated into shaking liquid cultures containing erythromycin or kanamycin. After overnight incubation (16 h) at 37°C, these inocula were determined with spectrophotometer at 570 nm.
A strain termed S. epidermidis RP62A△sarA (sarA::tet) derived from RP62A with an allele replacement of the sarA gene was obtained (Figure 1).
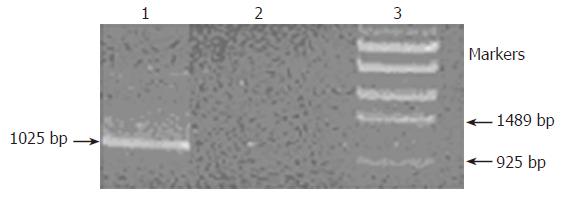
PCR analysis of DNA from strains RP62A and RP62A△sarA was performed with two primers as shown below: TB: 5’-ACGGAGCTCA AGCCTATGCC TACAGCA-3’ (in the central part of the tet gene) (Figure 1); SA2: 5’-ACGGAATTCG GGCATCATTG CGAGTGA-3’ (next to the downstream of sarA2 fragment) (Figure 1). The fragment was obtained as shown in Figure 2, which indicated that allelic replacement had taken place. The PCR-amplified fragment was further demonstrated by DNA sequencing, and the result revealed that a 1025 bp
fragment was composed of part of tet gene and chromosomal DNA (the datum of DNA sequence not shown).
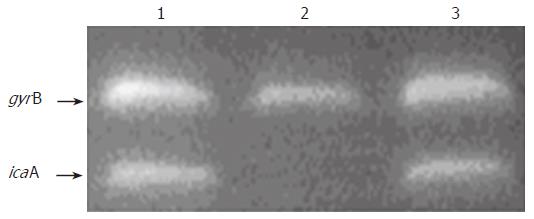
To investigate whether the transcription of ica operon expression was altered in the sarA mutant strain, RT-PCR was used to measure icaA transcription in variants grown in TSB. Total RNA of the original RP62A strain, its sarA mutant and corresponding complementary strain were isolated at early exponential and mid-log exponential phases, as the expression of ica operon was at maximum during this period. After treatment with DNase to remove contaminant DNA, RNA was reverse transcribed in the presence and absence of reverse transcriptase. The level of expression of icaA was normalized to gyrB expression[19]. Our results showed that the level of ica operon transcription was apparently reduced in the sarA mutation compared to that of the wild-type strain at exponential and mid-log exponential phases (Figure 3, Lane 2). Interestingly, in the sarA complementation strain, designated as RP62A△sarA (pHPS9sarA), the icaA transcription was activated (Figure 3, lane 3). Consistent with this, at the phenotypic level, the capacity of RP62A△sarA (pHPS9sarA) to form biofilm was restored in TSB. These data suggested that the gene of sarA in the strain of RP62A is responsible for activating ica operon expression.
Phenotypic assay on biofilm production: The S. epidermidis RP62A strain formed typical black, rough colonies after 24 h of incubation. The non-slime producing S. epidermidis ATCC 12228 formed smooth, red colonies. RP62A△sarA strain produced smooth, red colonies after 24 h, demonstrating the mutant was biofilm-negative. As to the RP62A△sarA (pHPS9sarA) strain, black, rough colonies phenotype was restored.
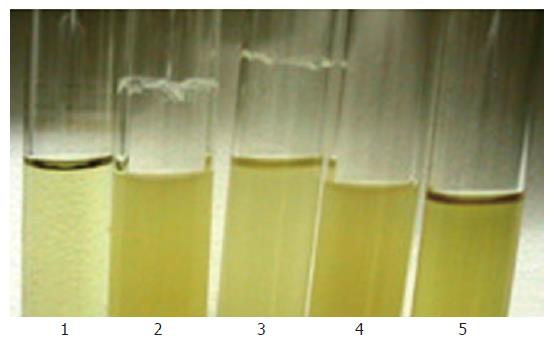
Biofilm formation on a glass surface of the shaking tube with overnight culture: The sarA mutant showed loss of the ability to produce a ring of biofilm adherent to the glass wall at the air-liquid interface (Figure 4, Lane 2). While the complementary strain RP62A△sarA (pHPS9sarA) displayed a biofilm positive phenotype similar to that of the wild-type strain (Figure 4, Lane 3).
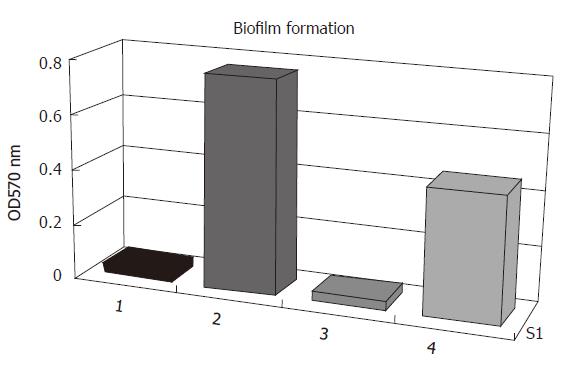
Quantitative determination of biofilm formation: All strains were then tested of their ability to form biofilms on polystyrene surfaces. The isolates were grown overnight in 96-well flat-bottomed tissue culture using TSB as growth medium. As shown in Figure 5, the biofilm formation of S. epidermidis RP62A was biofilm-positive when the strain was grown in TSB. In contrast, the S. epidermidis RP62A△sarA insertion mutant and S. epidermidis ATCC12228 failed to produce any detectable biofilm. In the case of S. epidermidis RP62A△sarA (pHPS9sarA), in which the deleted chromosomal locus of sarA was complemented by a plasmid carrying sarA, biofilm formation was restored.
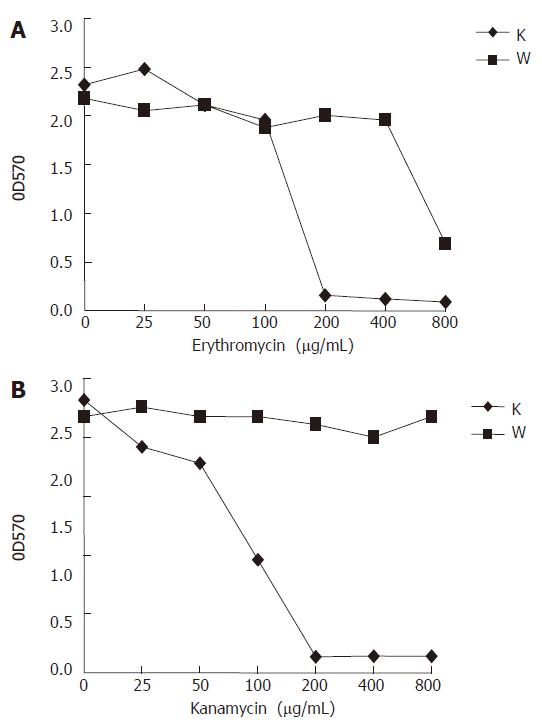
The sarA mutation had a dramatic effect on the antibiotics resistance of strain RP62A. Two antibiotics, erythromycin and kanamycin, were investigated. The sarA::tet mutant demonstrated a significant increase of susceptibility along with the increasing concentration of erythromycin and kanamycin compared to its parental strain (Figure 6). At erythromycin concentrations above 200 μg/mL, RP62A△sarA did not grow, while RP62A survived at concentrations over 800 μg/mL (Figure 6A). RP62A appeared at all kanamycin concentrations investigated, whereas RP62A△sarA did not survive at concentrations over 200 μg/mL (Figure 6B).
To investigate the impact of sarA on biofilm formation, we constructed a sarA mutant of S. epidermidis RP62A. At the phenotypic level, the sarA mutant revealed a biofilm-negative phenotype, and the capacity of biofilm formation was restored when sarA mutant strains were complemented by a plasmid carrying sarA. RT-PCR was used to measure the transcription of icaA in variants grown in TSB. Consistent with the biofilm-negative phenotype, the results showed that the sarA mutation caused a significant repression of the ica operon transcription compared with that of the wild-type strains. The absence of an identifiable sigB-consensus binding site on the upstream of the ica operon in S. aureus has suggested that sigB may not regulate ica operon directly[22], and the foundation of the SarA-consensus binding site implied that SarA controls directly the transcription of ica operon and subsequently regulates biofilm formation[23,24]. However, it is possible that both SigB and SarA are also involved in the posttranscriptional regulation of PIA synthesis in S. epidermidis according to previous reports.
The knockout of sarA resulted in decreased erythromycin and kanamycin resistance in S. epidermidis RP62A. This implies that a protein(s) whose production is controlled by SarA is involved in resistance to these antibiotics. However, the exact role that SarA plays in the response of S. epidermidis to multiple antibiotics deserves further attention.
In conclusion, the current studies demonstrate that SarA is typically associated with the transcription of the ica operon, the capacity of biofilm-formation and antibiotic resistance of S. epidermidis. To answer the question of whether SarA is directly or indirectly involved in those processes, more experimental work, including primer extension analyses under different growth conditions, is needed.
We thank professors Jiang Zhong and Han-Ying Yuan of Fudan University for their help with this study and Dr. Xiao-Ming Ding for revising the manuscript.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 785] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 2. | Fitzpatrick F, Humphreys H, O'Gara JP. The genetics of staphylococcal biofilm formation--will a greater understanding of pathogenesis lead to better management of device-related infection. Clin Microbiol Infect. 2005;11:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Mack D, Bartscht K, Dobinsky S, Horstkotte MA, Kiel K, Knobloch J, Schäfer P. Staphylococcal factors involved in adhesion and biofilm formation on biomaterials. Totowa: Humana Press 2000; 307-330. |
| 4. | Vadyvaloo V, Otto M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. Int J Artif Organs. 2005;28:1069-1078. [PubMed] |
| 5. | Nilsson M, Frykberg L, Flock JI, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666-2673. [PubMed] |
| 6. | Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 683] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 7. | Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427-5433. [PubMed] |
| 8. | Handke LD, Conlon KM, Slater SR, Elbaruni S, Fitzpatrick F, Humphreys H, Giles WP, Rupp ME, Fey PD, O'Gara JP. Genetic and phenotypic analysis of biofilm phenotypic variation in multiple Staphylococcus epidermidis isolates. J Med Microbiol. 2004;53:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Blevins JS, Beenken KE, Elasri MO, Hurlburt BK, Smeltzer MS. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect Immun. 2002;70:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Cheung AL, Bayer MG, Heinrichs JH. sar Genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963-3971. [PubMed] |
| 11. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed. the United States: Cold Spring Harbor Laboratory Press 1989; 1-57. |
| 12. | Kullik I I, Giachino P. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 480] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 653] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157370] [Article Influence: 3211.6] [Reference Citation Analysis (0)] |
| 16. | Gertz S, Engelmann S, Schmid R, Ziebandt AK, Tischer K, Scharf C, Hacker J, Hecker M. Characterization of the sigma(B) regulon in Staphylococcus aureus. J Bacteriol. 2000;182:6983-6991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Haima P, van Sinderen D, Bron S, Venema G. An improved beta-galactosidase alpha-complementation system for molecular cloning in Bacillus subtilis. Gene. 1990;93:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 245] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Conlon KM, Humphreys H, O'Gara JP. Regulation of icaR gene expression in Staphylococcus epidermidis. FEMS Microbiol Lett. 2002;216:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | O'Leary JO, Langevin MJ, Price CT, Blevins JS, Smeltzer MS, Gustafson JE. Effects of sarA inactivation on the intrinsic multidrug resistance mechanism of Staphylococcus aureus. FEMS Microbiol Lett. 2004;237:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:3357-3363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 22. | Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. Alternative transcription factor sigma(B) is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol. 2000;182:6824-6826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penadés JR, Lasa I. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol. 2003;48:1075-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Tormo MA, Martí M, Valle J, Manna AC, Cheung AL, Lasa I, Penadés JR. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J Bacteriol. 2005;187:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |