Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3983
Revised: January 20, 2006
Accepted: January 24, 2006
Published online: July 7, 2006
AIM: To investigate the inhibitory effects of a recombinant adenovirus vector that expresses NK4, a truncated form of human hepatocyte growth factor (HGF), on human colonic adenocarcinoma cells in vitro to establish a basis for future NK4 gene cancer therapy.
METHODS: Cells from the LS174T human colonic adenocarcinoma cell line were infected with recombinant adenovirus rvAdCMV/NK4 and the effects of the manipulation on tumor cell proliferation, scatter, migration, and basement membrane invasion were assessed. Cells infected with a recombinant adenovirus vector (Ad-LacZ) expressing β-galactosidase served as the controls.
RESULTS: We found that rvAdCMV/NK4 expression attenuated HGF-induced tumor cell scatter, migration, and basement membrane invasion (P < 0.05), but did not inhibit tumor cell proliferation.
CONCLUSION: HGF-induced LS174T tumor cell scatter, migration, and invasion can be antagonized by the recombinant NK4-expressing adenovirus.
-
Citation: Jie JZ, Wang JW, Qu JG, Wang W, Hung T. Effects of adenoviral-mediated gene transduction of NK4 on proliferation, movement, and invasion of human colonic LS174T cancer cells
in vitro . World J Gastroenterol 2006; 12(25): 3983-3988 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/3983.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.3983
Hepatocyte growth factor (HGF), a glycoprotein consisting of 728 amino acids with a 69 kD α-chain and a 34 kD β-chain, affects tumor metastasis[1,2]. HGF binds to c-Met, the only HGF receptor, and causes a cascade of enzyme catalyzed reactions, triggers signal transduction and the corresponding biological effects[3]. In the normal condition, the paracrine influence of HGF between the epithelial and interstitial cells is strictly controlled. However, when abnormal HGF/c-Met signaling occurs, such as there is a mutation of the c-Met gene, over-expression of HGF and/or c-Met, or there is co-expression of HGF and c-Met within the same cells, c-Met may be persistently over-expressed and thereby exhibit a high-level of self-phosphorylation. All these biological activities, in turn, facilitate the occurrence, growth, infiltration, metastasis, and angiogenesis of many kinds of tumors[4,5]. The HGF/c-Met signaling pathway has been thought to play an important role in the production of many kinds of human tumors[6,7]. Studies discovering the ways to block this pathway will provide new targets for the anti-metastasis tumor treatment.
NK4, a specific antagonist of HGF discovered by Date et al[8] in 1997, is a fragment from the HGF molecule that has been cut by elastase between the 478th and the 479th amino acid. NK4 derives its name from its structure: It is composed of the NH2-terminal hairpin domain and four subsequent kringle domains of the α-chain of HGF. NK4 contains 447 amino acids, and the molecular weight is about 50 kD. Although NK4 competes with HGF for c-Met receptor binding, it cannot activate the c-Met receptor and induce its phosphorylation. Consequently, NK4 suppresses the interaction between HGF and c-Met, interrupts the HGF/c-Met signaling pathway, and thereby inhibits HGF-induced invasion and metastasis of tumor cells[9,10].
In this study we used the replication-defective recombinant adenovirus rvAdCMV/NK4 expressing NK4 gene[11] to investigate the anti-tumor effects of NK4 in LS174T human colonic adenocarcinoma cells. The objective of this study was to explore whether NK4 may be used as a human colonic tumor treatment- a possibility that would require an experimental basis for the clinical application of manipulations of the NK4 gene product.
The construction and identification of the recombinant adenovirus rvAdCMV/NK4 with E1-E3 deletions have been described previously[11]. The recombinant adenovirus expressing β-galactosidase (Ad-LacZ) was generated in our laboratory using the same vector (data not published). These two viruses were replicated in a HEK293 human embryonic kidney cell line (American Type Culture Collection, Manassas, VA), which was maintained in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 100 mL/L fetal bovine serum (FBS) (Invitrogen). Culture titers were 8.9 × 1012 infection focus unite (ifu)/L and 6.4 × 1012 ifu/L, respectively. LS174T human colonic adenocarcinoma and MRC-5 human fetal lung fibroblast cell lines were purchased from the Shanghai Institute of Cell Biology at the Chinese Academy of Sciences and cultured in RPMI 1640 medium (Invitrogen) supplemented with 100 mL/L FBS, 100 U/mL penicillin, and 100 mg/L streptomycin (Invitrogen).
Total cellular proteins were extracted and aliquots were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The blots were incubated with rabbit anti-human c-Met polyclonal antibody [1:250] (Zymed Laboratories, San Francisco, CA) or goat anti-human HGF-α polyclonal antibody [1:500] (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at room temperature, followed by the addition of alkaline phosphatase (AP) conjugated secondary antibodies [1:5000] (Pierce, USA). AP was reacted with BCIP/NBT to visualize the bands.
LS174T cells were seeded in 24-well plates until they reached 80% confluency, at which time they were infected with 1, 10, 25, 50 or 100 multiplicity of infection (MOI) with Ad-LacZ for 48 h. The cells were washed with phosphate buffered saline (PBS) and fixed in 100 mL/L formaldehyde for 10 min. Fixed cells were then stained with X-gal overnight at 37°C. The number of positive cells in each well was counted by viewing under a light microscope. Transduction = (the number of positive cells/number of total cells) × 100%.
Two thousand five hundred LS174T cells/well were seeded in 96-well plates in triplicate and divided into a control group, an Ad-LacZ group, and a rvAdCMV/NK4 group. The cells were cultured for 24 h (d 0), then mock infected or infected with viruses at 50 MOI for 1 h. The medium was then replaced with RPMI 1640 containing 100 mL/L FBS, and the cells were further cultured and harvested on d 0, 1, 3, and 5. Cell proliferation was analyzed using the CellTiter 96 AQueous One Solution Regent (Promega, Madison, WI) according to the manufacturer’s protocol.
LS174T (2.5 × 103 cells/well) was seeded in 12-well plates. After culturing for 4 to 7 d at 37°C, the medium was replaced with 20 mL/L FBS RPMI 1640 medium. The cell cultures were divided into a control group, an Ad-LacZ group (50 MOI), and a rvAdCMV/NK4 group (50 MOI) and treated with recombinant human HGF (PeproTech EC, Rocky Hill, NJ) at a final concentration of 10 μg/L for 48 h. The cell colony scattering was observed and photographed.
Cell migration was measured by an in vitro scratch wound healing assay as described elsewhere[12]. Briefly, after LS174T cells were infected by 50 MOI of rvAdCMV/NK4 or Ad-LacZ for 48 h, the cells were trypsinized and adjusted to a density of 2 × 109 cells/L, then seeded in 12-well plates. Monolayer cells were scratched with a sterile pipette tip, and , the cell migration was evaluated by counting the number of cells that had migrated from the wound edge after culturing for 24 h in 20 mL/L FBS-supplemented RPMI 1640 medium.
Invasion assay was carried out as previously described[13]. Matrigel, an artificial matrix gel extracted from mice EHS sarcoma (purchased from the Department of Cellular Biology, Center for Health Sciences, Peking University, Beijing, China), was diluted by DMEM medium to
1 g/L. Polycarbonate membranes of the upper wells in a Transwell chamber (Corning Costar No. 3422) were loaded with 50 mL of Matrigel gel and exposed to UV for 30 min. Serum free medium was added to the chamber for polymerizing at 37°C for 30 min before using. LS174T cells that had been transduced with rvAdCMV/NK4 or Ad-LacZ at an MOI of 50 for 72 h were adjusted to a density of 1.5 × 109 cells/L. Cell suspension (100 μL) was seeded on the upper wells and 600 μL of serum-free RPMI 1640 media, with or without 10 μg/L HGF, was added to the lower wells of the Transwell chamber. After cultivating for 72 h, the cells that degraded from the Matrigel and migrated through the 8 μm pores of the membrane at the bottom of the upper wells to the opposite side of the membrane were stained with Hematoxylin and Eosin and counted. Five microscopic fields (× 200) were randomly selected for cell counting. The inhibitory rate = [(the number of invading cells in control group-the number in treated group)/the number of invading cells in control group] × 100%.
An inhibitory rate of more than 30%, P < 0.05, when compared with the group in which tumor cells in the upper wells and lower wells contained HGF, was considered to be evidence of an anti-invasion effect.
All data are expressed as means ± SD. Statistical comparisons were made using the Student’s t-test. P < 0.05 was considered significant.
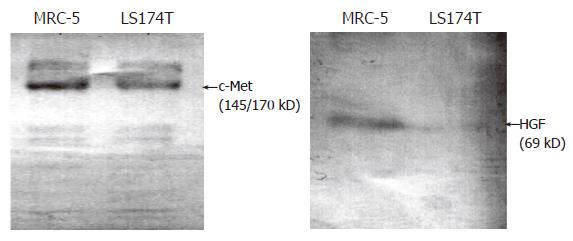
In this study we first examined the expression of c-Met, in order to test the reliability of using the colon cancer cell line LS174T as a study model. Western blot results showed that two specific bands were detectable in both LS174T and MRC-5 (Control): One was the 145 kD β-subunit of c-Met protein and the other was the 170 kD c-Met protein existing in the heterogeneous dimmer form. This finding suggests that LS174T cells could be used as target cells of HGF and thus employed in further experimental studies. Expression of HGF was detected in MRC-5 cells, but was not detected in LS174T cells (Figure 1).
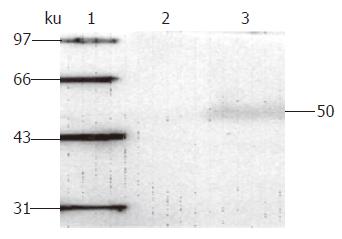
In order to optimize the gene transduction mediated by the adenovirus, we used the recombinant adenovirus Ad-LacZ, containing the report gene β-galactosidase, to infect LS174T cells at different MOI. We found that the infection rate increased with increasing MOI in a dose-dependent manner (data not shown). When the infection intensity reached 50 MOI, Ad-LacZ achieved a LS174T cell infection rate of more than 90% without pathological changes. Since overdose recombinant adenovirus infection will hurt cells and disturb cell growth, the optimal infection dose in all of our experiments was set as 50 MOI. rvAdCMV/NK4 infection of LS174T cells was confirmed by Western blot analysis. A specific NK4 protein band at 50 kD was detected, demonstrating that efficient expression of the NK4 gene mediated by the adenovirus was achieved in the LS174T cells (Figure 2). Thus, rvAdCMV/NK4-infected LS174T cells were found to be suitable for our experiments.
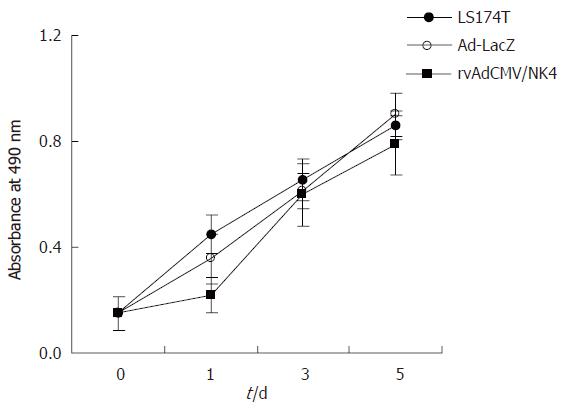
We measured the effect of rvAdCMV/NK4 transduction on proliferation of LS174T cells. The proliferation rate of the tumor cells infected by rvAdCMV/NK4 was similar to that of the control group and the Ad-LacZ group (P > 0.05). As summarized in Figure 3, these data suggest that expression of the NK4 gene mediated by the adenovirus had no suppressive effect on LS174T cell proliferation.
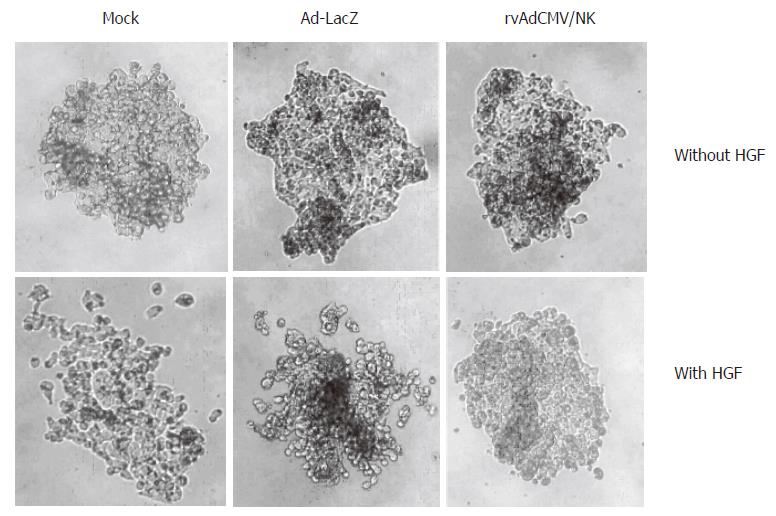
Since HGF facilitates cell colony scatter, we designed this experiment to determine whether NK4 has an inhibitory effect on cell scattering. The results showed that intercellular adhesion among untreated LS174T cell colonies grown for 4 to 7 d was strong and that cells were not prone to scatter. But after HGF treatment, adhesion was relatively weak and the cells scattered in a spider-like pattern. In contrast, this HGF-effect was inhibited in rvAdCMV/NK4-infected cells. However, sole Ad-LacZ or rvAdCMV/NK4 infection did not influence intercellular adhesion, indicating that NK4 could specifically antagonize HGF-induced cell scattering (Figure 4).
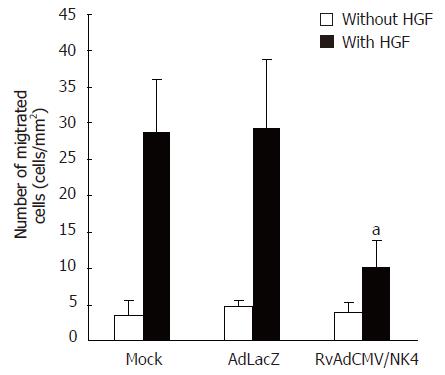
We performed a scratch wound healing assay to determine whether NK4 expressing recombinant adenovirus could attenuate HGF-induced cell motility. Without HGF treatment, the number of Ad-LacZ or rvAdCMV/NK4 infected LS174T cells that migrated into the scratch wound area were similar with that of uninfected LS174T cells. However with HGF treatment, we observed that less NK4-expressing cells migrated to the scratch wound area than control cells did, indicating that HGF activity of promoting tumor cell movement was antagonized by NK4. The number of cells migrating into the scratch wound areas of each group after HGF treatment was: 28.8 ± 7.1/mm2 in the control group; 29.3 ± 9.4/mm2 in the Ad-LacZ group (P > 0.05 vs control group); and 10.2 ± 3.6/mm2 in the rvAdCMV/NK4 group (P < 0.05 vs control group, Figure 5). These results suggest that the recombinant NK4 expressing adenovirus selectively antagonized HGF-induced cell movement.
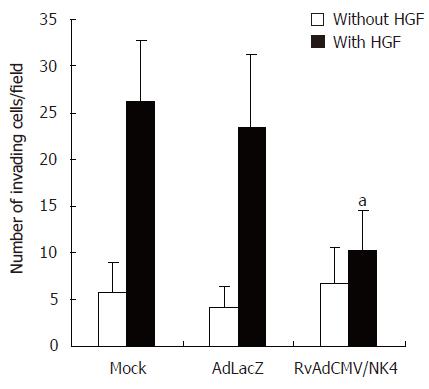
Tumor cell invasion of the basement membrane is a key process during metastasis. Matrigel, which is a kind of artificial matrix gel extracted from mouse EHS sarcoma, forms a membrane structure similar to the natural basement membrane structure in serum-free medium. The malignant invasion capacity of cells is revealed by their ability to penetrate into the filter membrane under the influence of an inductive chemo-attractant. The influence of specific factors on tumor cell invasive capacity can be observed in vitro by counting the number of cells that penetrate the filter membrane. The ability of untreated LS174T cells to degrade and penetrate the basement membrane was poor, but was improved when HGF was present in the lower chamber. The number of Ad-LacZ or rvAdCMV/NK4 infected LS174T cells that penetrated through the membrane without HGF in the lower chamber was similar to that of non-infected LS174T cells (P > 0.05). However, the invasive capacity of LS174T cells decreased significantly when HGF was present in the lower chamber (inhibitory rate 71.6%) after infection with rvAdCMV/NK4; meanwhile the inhibitory rate was only 7.3% in the Ad-LacZ treated group. This difference (P < 0.05) suggests that NK4 can specifically inhibit HGF-induced LS174T cell invasion (Figure 6).
Despite considerable advances in anesthesiology and surgical techniques as well as the development of new radiotherapy and chemotherapy strategies, the survival rate associated with colonic cancer has not been greatly improved. Tumor metastasis, including local metastasis to peripheral viscera and distant metastasis, is thought to be an important factor in determining survivability. It is reported that 15% to 35% of patients suffering from primary colonic cancer had liver metastasis when their primary tumors were diagnosed. Even among those patients who accept radical treatment for colonic cancer, 25% will have liver metastasis[14]. Accordingly, the prognosis of patients suffering from colonic cancer is primarily associated with the occurrence of metastasis, especially to the liver. Thus inhibition of metastasis will markedly improve the prognosis of colon cancer patients.
Changes in oncogenes and anti-oncogenes within cells contribute to tumorigenesis, but tumor metastasis behavior is primarily regulated by extracellular activated growth factors. Stroma cells can regulate tumor cell motility by releasing various cytokines, and consequently may initiate tumor metastasis[15-17]. HGF is one such important factor, released primarily by stroma fibroblasts, and initially identified and cloned as a potential liver cell mitogen[18,19]. HGF has also been found to facilitate cell movement and extracellular matrix breakthrough, resulting in the scatter of many kinds of tumor cells[4,20-22] and stimulating tumor cell proliferation and stroma cell angiogenesis[23]. All of the effects of HGF are realized through its binding with the c-Met receptor, and c-Met receptors are over-expressed in many kinds of tumor cells[24-26]. In this study we used the recombinant NK4 expressing adenovirus to infect the LS174T human colonic cancer cell line. Our results showed that NK4 could efficiently antagonize the effects of HGF, that is, it could inhibit the migration, scatter, and invasion of tumor cells. This suggests that NK4 is capable of blocking the HGF/c-Met signaling pathway and thereby may play an important role in antagonizing the invasion and metastasis of colonic cancer.
Highly malignant LS174T cells were harvested from poorly differentiated epithelial adenocarcinoma tissue. Western blot analysis indicated that c-Met protein was expressed in the LS174T cells. These findings demonstrate the feasibility of using recombinant adenovirus expressing NK4 to block the HGF/c-Met signaling pathway as a clinical intervention for colonic cancer. We did not detect HGF expression in the LS174T cells; however, the c-Met receptor was triggered clearly after HGF treatment, producing enhanced scatter, motility and invasion. These findings indicate that the HGF treatment was sufficient for this study. In in vitro cell proliferation experiments, rvAdCMV/NK4 had no effect on tumor cell proliferation. This is consistent with the studies of Kubota et al[27] and Saimura et al[28] indicating that the virus itself does not influence scatter and invasion.
A close relationship between the ability of tumor cells to move and their invasion capacity has been demonstrated[29]. Thus suppression of tumor cell movement would be expected to inhibit invasion and metastasis[30]. Initially, tumor cell movement appears as a scattering of the cells away from one another. We used a scatter assay and a scratch wound healing assay to reveal the inhibitory effects of NK4 on HGF. HGF reduced the intercellular homogeneous adhesion of LS174T cells; such as LS174T cells scattered and migrated. These effects were inhibited by rvAdCMV/NK4; however rvAdCMV/NK4 alone (without the presence of HGF) did not have any biological effects on LS174T cells, indicating that the effects of NK4 were specific for the HGF signal. The findings of Parr et al[31] was shown that the growth factor TGF-β was able to facilitate tumor cell scattering in the presence of the NK4 protein and provide further evidence for the conclusion that NK4 specifically suppresses the influence of HGF.
Once tumor cells fall off the original tumors they invade the extracellular matrix of the host, and then penetrate the lymphatic system or the blood vessels, resulting in distant metastasis. The Matrigel material is similar to the extracellular matrix and therefore can be used to simulate tumor cell penetration into the cellular matrix around blood vessels. LS174T cells were able to degrade the Matrigel and migrate across the 8 μm membrane pores under the inductive influence of the chemo-attractant HGF. rvAdCMV/NK4 weakened the HGF-induced cell capacity for penetrating the basement membrane, but rvAdCMV/NK4 alone did not produce this effect. Dormancy therapy aimed at preventing angiogenesis has become a hot topic of discussion regarding with anti-tumor treatment[32]. We did not investigate the antagonizing effect of NK4 on HGF-induced angiogenesis, which plays an important role in tumorigenesis and distant metastasis. However, recent studies have shown that the anti-angiogenesis effects produced by NK4 appear to be the result of not only a blockade of HGF-mediated effects, but also a blockade of effects mediated by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)[33,34]. Thus NK4 may be considered as a multi-potential anti-tumor therapeutic protein. Because NK4 has the potential to impact many aspects of tumorigenesis, it will be great applicable in the future clinical treatment of tumors.
In conclusion, this study demonstrated that adenoviral-mediated NK4 inhibits the invasion and metastasis of LS174T colonic cancer cells by blocking the HGF/c-Met signaling pathway in vitro. These findings establish an experimental basis for further studies in vivo.
S- Editor Pan BR L- Editor Zhao JB E- Editor Bai SH
| 1. | Matsumoto K, Nakamura T. HGF-c-Met receptor pathway in tumor invasion-metastasis and potential cancer treatment with NK4. Growth factors and their receptors in cancer metastasis. Dordrecht: Kluwer Academic Publisher 2001; 241–276. |
| 2. | Jiang W, Hiscox S, Matsumoto K, Nakamura T. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit Rev Oncol Hematol. 1999;29:209-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Stuart KA, Riordan SM, Lidder S, Crostella L, Williams R, Skouteris GG. Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int J Exp Pathol. 2000;81:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Stella MC, Comoglio PM. HGF: a multifunctional growth factor controlling cell scattering. Int J Biochem Cell Biol. 1999;31:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | van der Voort R, Taher TE, Derksen PW, Spaargaren M, van der Neut R, Pals ST. The hepatocyte growth factor/Met pathway in development, tumorigenesis, and B-cell differentiation. Adv Cancer Res. 2000;79:39-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Jeffers M, Rong S, Vande Woude GF. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med (Berl). 1996;74:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Longati P, Comoglio PM, Bardelli A. Receptor tyrosine kinases as therapeutic targets: the model of the MET oncogene. Curr Drug Targets. 2001;2:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Haddad R, Lipson KE, Webb CP. Hepatocyte growth factor expression in human cancer and therapy with specific inhibitors. Anticancer Res. 2001;21:4243-4252. [PubMed] |
| 10. | Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem Biophys Res Commun. 2005;333:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Jie JZ, Wang JW, Wang W, Jiang XL, Wei Q, Hung T. Expression and characterization of HGF mutant NK4 in adenovirus vector. Zhongguo Shiyan Zhenduanxue. 2003;7:397-400. |
| 12. | Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 924] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 13. | Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239-3245. [PubMed] |
| 14. | Calaluce R, Miedema BW, Yesus YW. Micrometastasis in colorectal carcinoma: a review. J Surg Oncol. 1998;67:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Birchmeier C, Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Sakakura T. New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol. 1991;125:165-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hu M, Pollock RE, Nakamura T, Nicolson GL. Human peri-tumoral and lung fibroblasts produce paracrine motility factors for recently established human sarcoma cell strains. Int J Cancer. 1995;62:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 830] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1628] [Cited by in RCA: 1626] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 20. | Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 439] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Vande Woude GF, Jeffers M, Cortner J, Alvord G, Tsarfaty I, Resau J. Met-HGF/SF: tumorigenesis, invasion and metastasis. Ciba Found Symp. 1997;212:119-130; discussion 130-132, 148-154. [PubMed] |
| 22. | Matsumoto K, Nakamura T. NK4 (HGF-antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci. 2003;94:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Maemondo M, Narumi K, Saijo Y, Usui K, Tahara M, Tazawa R, Hagiwara K, Matsumoto K, Nakamura T, Nukiwa T. Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther. 2002;5:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997-2003. [PubMed] |
| 25. | Kaji M, Yonemura Y, Harada S, Liu X, Terada I, Yamamoto H. Participation of c-met in the progression of human gastric cancers: anti-c-met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther. 1996;3:393-404. [PubMed] |
| 26. | Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M, Nakamura T. NK4, a four-kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br J Cancer. 2001;84:864-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Kubota T, Fujiwara H, Amaike H, Takashima K, Inada S, Atsuji K, Yoshimura M, Matsumoto K, Nakamura T, Yamagishi H. Reduced HGF expression in subcutaneous CT26 tumor genetically modified to secrete NK4 and its possible relation with antitumor effects. Cancer Sci. 2004;95:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Saimura M, Nagai E, Mizumoto K, Maehara N, Okino H, Katano M, Matsumoto K, Nakamura T, Narumi K, Nukiwa T. Intraperitoneal injection of adenovirus-mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC-1 in nude mice. Cancer Gene Ther. 2002;9:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Grimstad IA. Direct evidence that cancer cell locomotion contributes importantly to invasion. Exp Cell Res. 1987;173:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856-1862. [PubMed] |
| 31. | Parr C, Hiscox S, Nakamura T, Matsumoto K, Jiang WG. Nk4, a new HGF/SF variant, is an antagonist to the influence of HGF/SF on the motility and invasion of colon cancer cells. Int J Cancer. 2000;85:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Ramanujan S, Koenig GC, Padera TP, Stoll BR, Jain RK. Local imbalance of proangiogenic and antiangiogenic factors: a potential mechanism of focal necrosis and dormancy in tumors. Cancer Res. 2000;60:1442-1448. [PubMed] |
| 33. | Ueda K, Iwahashi M, Matsuura I, Nakamori M, Nakamura M, Ojima T, Naka T, Ishida K, Matsumoto K, Nakamura T. Adenoviral-mediated gene transduction of the hepatocyte growth factor (HGF) antagonist, NK4, suppresses peritoneal metastases of gastric cancer in nude mice. Eur J Cancer. 2004;40:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Nakabayashi M, Morishita R, Nakagami H, Kuba K, Matsumoto K, Nakamura T, Tano Y, Kaneda Y. HGF/NK4 inhibited VEGF-induced angiogenesis in in vitro cultured endothelial cells and in vivo rabbit model. Diabetologia. 2003;46:115-123. [PubMed] |