Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3965
Revised: March 15, 2006
Accepted: March 21, 2006
Published online: July 7, 2006
AIM: To study the expression of Sonic hedgehog pathway-related molecules, Sonic hedgehog (Shh) and Gli1 in gastric carcinoma.
METHODS: Expression of Shh in 56 gastric specimens including non-cancerous gastric tissues, gastric adenocarcinoma, gastric squamous cell carcinoma was detected by RT-PCR, in situ hybridization and immunohistochemistry. Expression of Gli1 was observed by in situ hybridization.
RESULTS: The positive rate of Shh and Gli1 expression was 0.0%, 0.0% in non-cancerous gastric tissues while it was 66.7%, 57.8% respectively in gastric adenocarcinoma, and 100%, 100% respectively in gastric squamous cell carcinoma. There was a significant difference between the non-cancerous gastric tissues and gastric carcinoma (P < 0.05). Elevated expression of Shh and Gli1 in gastric tubular adenocarcinoma was associated with poorly differentiated tumors while the expression was absent in gastric mucinous adenocarcinoma.
CONCLUSION: The elevated expression of Shh and Gli1 in gastric adenocarcinoma and gastric squamous cell carcinoma shows the involvement of activated Shh signaling in the cellular proliferation of gastric carcinogenesis. It suggests Shh signaling gene may be a new and good target gene for gastric tumor diagnosis and therapy.
- Citation: Ma XL, Sun HJ, Wang YS, Huang SH, Xie JW, Zhang HW. Study of Sonic hedgehog signaling pathway related molecules in gastric carcinoma. World J Gastroenterol 2006; 12(25): 3965-3969
- URL: https://www.wjgnet.com/1007-9327/full/v12/i25/3965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.3965
Sonic hedgehog (Shh), a mammalian homologue of Drosophila secreted morphogen Hedgehog, is crucial for the development of various embryonic tissues in invertebrate and vertebrate development, including brain, spinal cord, axial skeleton, limbs, lungs, gut, and hematopoietic cells[1-4]. Shh is synthesized in epithelial cells. Its membrane receptor Patched1 (Ptc1) is expressed in adjacent mesenchymal cells. Ptc1, a 12-transmembrane protein, does not transduce the intracellular signals by itself. This task is executed by smoothened (Smo), a 7-transmembrane protein that belongs to heterotrimeric G protein-coupled receptor (GPCR) family. In the absence of Shh, Ptc1 suppresses the activity of Smo by binding to Smo. Upon Shh stimulation, Shh binds to Ptc1, and then Smo is de-repressed from the suppression of Ptc1 and is able to transduce the intracellular signals to transcriptor Gli. Gli transfers the signals into the nucleus[5].
Shh is implicated in the early expansion of developing midbrain and also in the proliferation of granular cell precursors in the cerebellum[6-8]. In human, likewise in experimental models, the activated Shh signaling is thought to predispose to the development of tumors[9-12]. The role of the Shh pathway in the regulation of oncogenic transformation is a new and exciting field. The study by Berman et al suggests that this pathway may well offer the potential for new treatments for medulloblastoma[11]. The recent finding that Shh pathway activity is important for growth of small cell lung cancers, a tumor type not associated with Gorlin’s syndrome, suggested that other, non-Gorlin’s tumors might require Shh pathway activity for growth[12-14]. In the longer term, better understanding of the regulatory role of this pathway may offer new targets for therapeutic manipulation.
Gastric cancer is the second most common cause of cancer-related death in the world. Many Asian countries, including Korea, China, and Japan, have very high rates of gastric cancer[15]. By far, the mechanism of Hedgehog signal in gastric cancer is still unclear. Here we studied the role of Shh signaling pathway-related molecules, Shh and Gli1, in gastric adenocarcinoma and squamous cell carcinoma by RT-PCR, immunohistochemistry and in situ hybridization.
A total of 56 specimens of gastric tissues were used in our study. Fifty-six patients, who had undergone curative gastrectomy between 2002 and 2005 from the Department of General Surgery, Shandong Qilu Hospital affiliated to Shandong University, or from Jinan Central Hospital, Shandong Province, China, were enrolled in this study. All of the resected primary tumors were histologically examined by hematoxylin and eosin staining,and confirmed by pathologists. These samples included five resection specimens of non-cancerous gastric tissues, ten well differentiated tubular adenocarcinoma, ten moderately differentiated tubular adenocarcinoma, fifteen poorly differentiated adenocarcinoma, seven papillary adenocarcinoma, three gastric mucinous adenocarcinoma and six gastric squamous cell carcinoma.
Representative formalin fixed and paraffin embedded tissue sections (6 μm thick) were used for immunohistochemistry with specific antibodies to human Shh (Cat# 9024, Santa Cruz Biotechnology Inc, Santa Cruz, CA). First, tissue sections were deparaffinized, followed by rehydration with serially decreased concentrations of ethanol, and immersed in 3% H2O2 (in distilled water) for 10 min to inhibit endogenous peroxidase activity. Following antigen retrieval in citrate buffer (pH 6.0), the tissue sections were incubated with normal goat serum to block nonspecific antibody binding for 20 min at room temperature. The sections were then incubated with primary antibodies (at 1:200 dilution) at 37°C in humid chambers for 2 h. After washing with PBS 3 times, the sections were incubated with biotinylated secondary antibody (goat anti-rabbit IgG) and streptavidin conjugated to horseradish peroxidase for 20 min at 37°C, followed by PBS wash. The sections were incubated with DAB substrate for less than 30 min. Haematoxylin was used for counterstaining. Negative controls were performed in all cases by omitting the first antibodies.
Total RNA of cells was extracted by an using RNA extraction kit (Promega). Total RNA templates were isolated from 10 μg sections (10 μm) of paraffin-embedded tissue as described elsewhere[16]. Three micrograms of total RNA were reverse transcribed by using M-MLV reverse transcriptase (Promega) with mixture of oligo(dT)15 and random primers (Promega). One-tenth of each RT reaction mixture was then subjected to PCR amplification using Taq DNA polymerase (TAKARA). The PCR primers for detecting specific transcripts are as below: for Shh sense 5’-ACCGAGGGCTGGGACGAAGA-3’ and antisense 5’-ATTTGGCCGCCACCGAGTT-3’ respectively. Following denaturation at 94°C for 10 min, 35 PCR cycles were performed at 94°C for 60 s, at 52°C for 50 s, and at 72°C for 60 s. The PCR products were analyzed by 0.7% agarose gel electrophoresis.
Shh (L38518) was subcloned in PbluescriptKS+. The linearized pBlueScript-shh was obtained by digestion with Sph I or Xmn I restriction endonuclease. Gli1 (X07384) was cloned into pBluescript M13+KS. The plasmid was digested with Nru I to generate the sense fragment, with Nde I to generate the antisense fragment. Sense and antisense probes were obtained by T3 and T7 in vitro transcription using a kit from Roche (Mannheim, Germany). Tissue sections (6 μm thick) were mounted onto Poly-L-Lysine slides. Following deparaffinization, tissue sections were rehydrated in a series of dilutions of ethanol. To enhance signal and facilitate probe penetration, sections were immersed in 0.3% Triton X-100 solution for 15 min at room temperature and in proteinase K (2 mg/mL) solution for 20 min at 37°C, respectively. The sections were then incubated with 4% (v/v) paraformaldehyde/PBS for 5 min at 4°C. After washing with PBS and 10 × saline citrate, the slides were incubated with prehybridization solution (50% formamide, 50% 4 × SSC) for 2 h at 37°C. The probe was added to each tissue section at a concentration of 1 g/mL and hybridized overnight at 42°C. After high stringency washing (2 × SSC twice, 1 × standard saline citrate twice, 0.5 × SSC twice at 52°C), sections were incubated with an alkaline phosphatase-conjugated sheep anti-digoxigenin antibody, which catalyzed a color reaction with the NBT/BCIP (nitro-blue-tetrazolium/5-bromo-4-chloro-3-indolyl phosphate) substrate (Roche, Mannheim, Germany). Blue indicated strong hybridization. As negative controls, sense probes were used in all hybridization and no positive signal was observed.
Analysis was performed by using chi-square test and correlation analysis with SPSS 11.0 software. P < 0.05 was considered statistically significant.
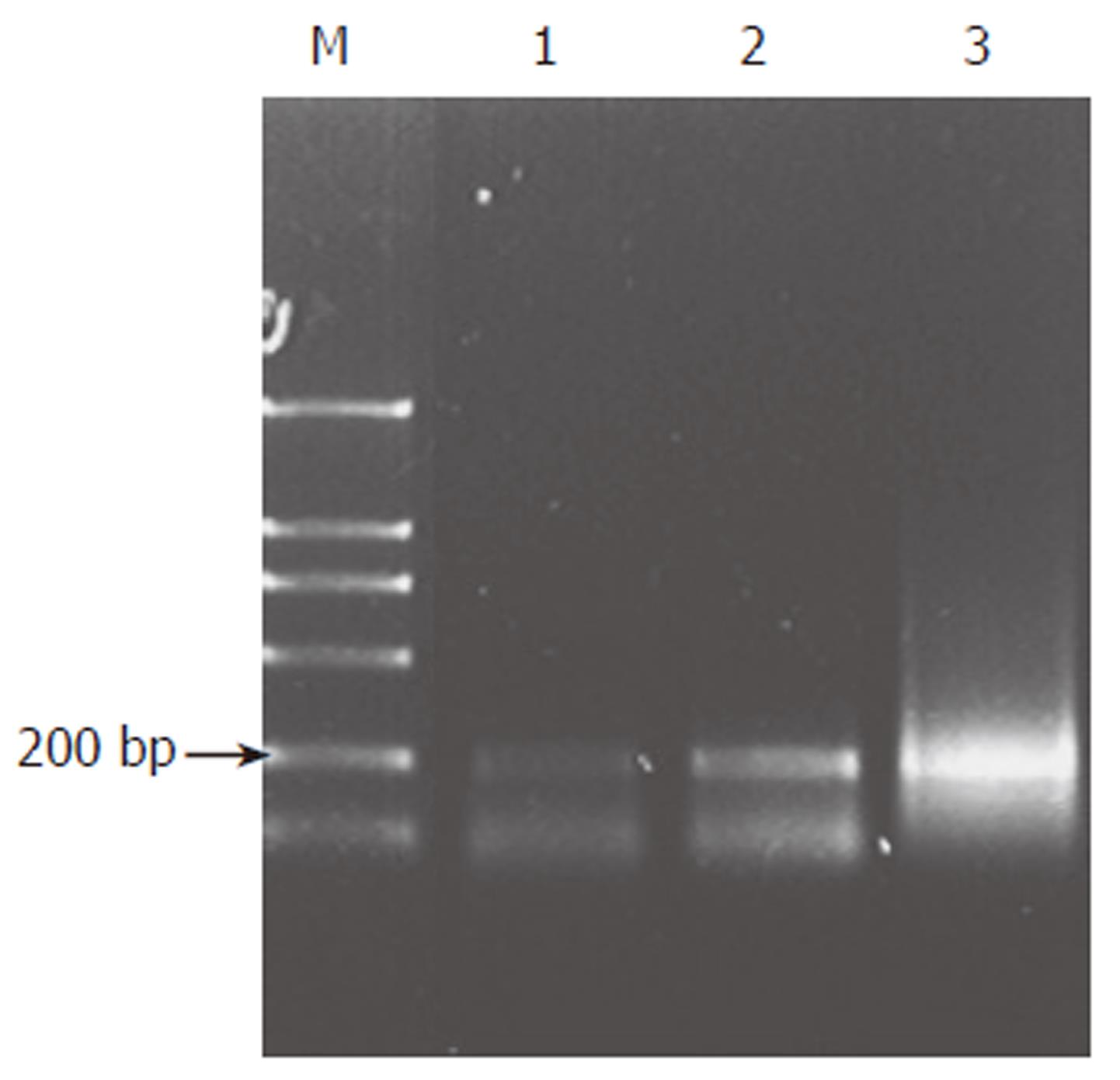
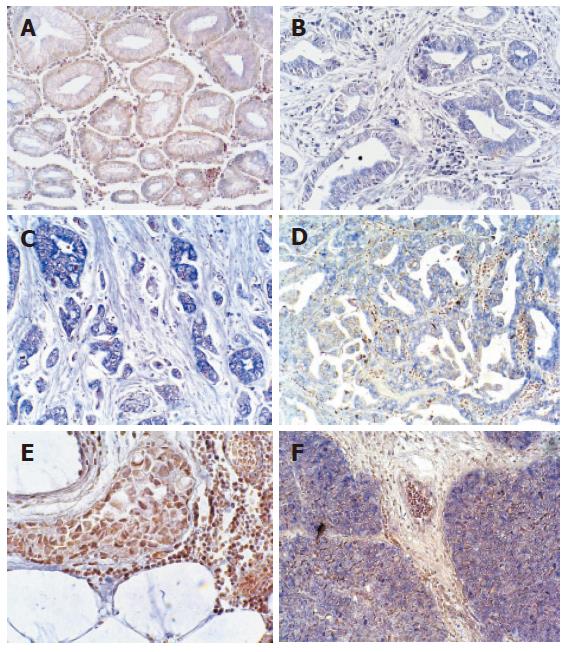
Total RNA extracted in paraffin-embedded tissue was pure enough to meet the need for RT-PCR. The length of RT-PCR product was 211 bp. The results of RT-PCR showed that expression of Shh was different in various tissue, including non-cancerous gastric tissues, well differentiated and poorly differentiated adenocarcinoma. Expression of Shh was higher in gastric adenocarcinoma than in non-cancerous gastric tissues (Figure 1), which was confirmed by in situ hybridization. The results of in situ hybridization showed that Shh mRNA was expressed in cytoplasm of the fundic glandular epithelium and some stromal cells. Shh was not or lowly expressed in non-cancerous gastric tissues’ glandular epithelium while overexpressed in about 66.7% (30/45) adenocarcinoma patients and in 100% (6/6) gastric squmaous cell carcinoma (Figure 2A-F) (Table 1).
| Tumor (n) | Expression of Shh n (%) | Expression of Gli1 n (%) | P value | |
| Non-cancerous gastric tissues | 5 | 0 (0.0) | 0 (0.0) | 0.01 < P < 0.05 |
| Adenocarcinoma | 45 | 30 (66.7) | 26 (57.8) | |
| Tubular | ||||
| Well differentiated (WD) | 10 | 4 (40.0) | 3 (30.0) | |
| Moderately differentiated (MD) | 10 | 8 (80.0) | 7 (70.0) | |
| Poorly differentiated (PD) | 15 | 13 (86.7) | 12 (80.0) | |
| Papillary | 7 | 5 (71.4) | 4 (57.1) | |
| Mucinous | 3 | 0 (0.0) | 0 ( 0.0) | |
| Squamous cell carcinoma | 6 | 6 (100) | 6 (100) | |
In tubular and papillary adenocarcinoma glandular epithelium, the expression of Shh showed an increasing trend among well differentiated, moderately differentiated and poorly differentiated adenocarcinomas (Figure 2B-D), while expression of Shh was absent in gastric mucinous adenocarcinoma (Figure 2E). In gastric squamous cell carcinoma, expression of Shh was obvious (Figure 2F). There was a significant difference between the non-cancerous gastric tissues and gastric carcinoma (P < 0.05) (Table 1).
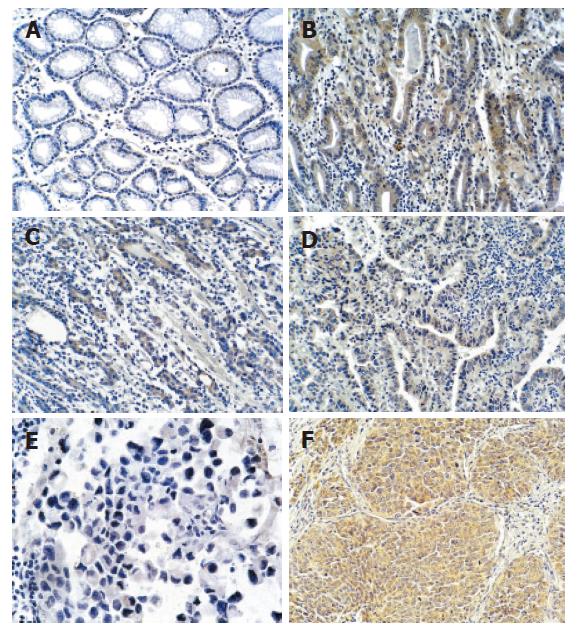
The results of immunohistochemistry showed that Shh staining was detected in the fundic glandular epithelium and in the stroma of the stomach (Figure 3A-F). Shh protein was not or lowly expressed in non-cancerous gastric tissues (Figure 3A) and was highly expressed in gastric adenocarcinoma and gastric squamous cell carcinoma (Figure 3B-D, F). The results of Shh expression in various gastric tissue were consistent with results of in situ hybridization. Shh was overexpressed in gastric adenocarcinoma epithelium and squamous cell carcinoma epithelium compared with non-cancerous stomach tissues glandular epithelium. Shh expression was increased with malignant degree aggravation in gastric adenocarcinoma (Figure 3B-D), while there was no or less expression of Shh in gastric mucinous adenocarcinoma (Figure 3E).
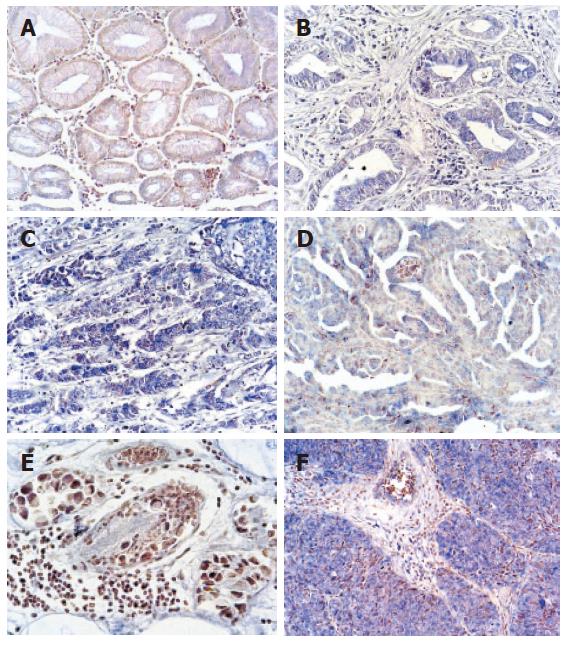
As an Shh signaling transcriptor, Gli1, was detected by in situ hybridization. It showed Gli1 was not or lowly expressed in non-cancerous gastric tissues glands and highly expressed in epithelium cytoplasm, in 57.8% (26/45) gastric adenocarcinoma and 100% (6/6) squamous cell carcinoma (Figure 4A-F) (Table 1). There was a higher expression level of Gli1 in squamous cell carcinoma (Figure 4F). Also, it presented an increasing trend in tubular adenocarcinoma with malignant degree aggravation (Figure 4B-D), while there was no or lower expression of Shh in gastric mucinous adenocarcinoma (Figure 4E). There was a significant difference between the non-cancerous gastric tissues and gastric carcinoma (P < 0.05) (Table 1).
Shh is an important endodermal signal in endodermal-mesenchymal interaction during vertebrate development of the gut tube, especially to gastric gland[17]. Because Shh is expressed consistently in the endodermal epithelium of the gut throughout the period of organogenesis and late embryonic life, it is natural to suppose that its expression is crucial for the differentiation and maintenance of gut tube epithelium. Indeed, the heterozygous mutant mouse for Shh shows various gastrointestinal defects, such as intestinal transformation of stomach, annular pancreas, and duodenal stenosis[18]. Some of these defects may be caused by the absence of Shh effects on the mesenchyme, while others may reflect a direct action of Shh on epithelial cells. Although the expression of Shh is essential for the development of gut tube and the fundic glands of the adult gastrointestinal tract, the role of Shh expression has not been described in the gastric carcinogenesis[14,15]. We examined Sonic hedgehog signaling pathway related molecules expression in non-cancerous gastric tissues and in various types of gastric carcinoma. The results indicated that Shh protein expression is closely correlated with glandular epithelium differentiation in adenocarcinoma and gastric squamous cell carcinoma. The increase of Shh expression in tumor tissue is consistent with the results of Berman et al[14].
Gastric cancer is one of the leading causes of cancer death worldwide. Because of its heterogeneity, gastric cancer has been an interesting model for studying carcinogenesis and tumorigenesis[15]. This study mainly detected the expression of Shh and Gli1 in various types of gastric carcinoma. The elevated expression of Shh and Gli1 in gastric adenocarcinoma and squamous cell carcinoma suggested the involvement of activated Shh signaling in the cellular proliferation of certain type of gastric carcinoma. Interestingly, there was no activation of Shh signaling in mucinous adenocarcinoma. It implies that there may exist different mechanisms in various gastric carcinoma.
In all the tumors with elevated levels of Gli1, expression of Shh was also increased, suggesting that overexpression of Shh may be responsible for elevated expression of Gli1 in gastric adenocarcinoma. However, a high level of sonic hedgehog expression was not always accompanied by elevated Gli1 expression in adenocarcinoma (Table 1), indicating additional regulatory mechanisms for the hedgehog pathway activation. In addition to the hedgehog overexpression, other genetic alterations may be required to activate the hedgehog pathway in gastric cancers, such as Wnt, Notch signal pathway. Further study of the relationship between Sonic Hedgehog and other signal pathways may provide more evidences for a new and good target gene for gastric cancer diagnosis and therapy.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bi L
| 1. | Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2382] [Cited by in RCA: 2331] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 2. | Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 516] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 451] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2337] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 5. | Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Britto J, Tannahill D, Keynes R. A critical role for sonic hedgehog signaling in the early expansion of the developing brain. Nat Neurosci. 2002;5:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1014] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 8. | Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055-9067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 420] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Katano M. Hedgehog signaling pathway as a therapeutic target in breast cancer. Cancer Lett. 2005;227:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Hahn H, Wojnowski L, Miller G, Zimmer A. The patched signaling pathway in tumorigenesis and development: lessons from animal models. J Mol Med (Berl). 1999;77:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 613] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 13. | Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1158] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 14. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 967] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 15. | Dimmler A, Brabletz T, Hlubek F, Häfner M, Rau T, Kirchner T, Faller G. Transcription of sonic hedgehog, a potential factor for gastric morphogenesis and gastric mucosa maintenance, is up-regulated in acidic conditions. Lab Invest. 2003;83:1829-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Ma XL, Huang AH, Wang YS, Xie JW, Zhang HW. Study of RNA extracted from paraffin tissues. Shandong Daxue Xuebao (Yixue Ban). 2004;42:613-614. |
| 17. | van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763-2772. [PubMed] |