Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3901
Revised: January 10, 2006
Accepted: January 14, 2006
Published online: June 28, 2006
AIM: To evaluate the frequency of the loss of the Adenomatous Polyposis Coli (APC) protein and to compare the APC status with the characteristics of colorectal adenomas.
METHODS: Immunohistochemical analysis of the APC protein was performed on 118 adenomas and the results were compared with parameters of malignant potential, location of adenomas, macroscopic appearance and age of the patients.
RESULTS: A complete loss of the APC protein was found in 28 (24%) adenomas, while 90 (76%) were APC positive. The mean size of adenomas was 13.5 ± 14.2 mm (95% CI 10.5-16.5) in APC-positive, and 13.8 ± 15.5 mm (95% CI 7.8-19.8) in APC-negative adenomas (P = 0.364). Statistical analysis revealed no difference between APC-positive and negative adenomas as to the histological type (P = 0.327) and grade of dysplasia (P =0.494). We found that even advanced adenomas did not differ in their APC status from the non-advanced tumors (P = 0.414). Finally, no difference was found when the location (P = 0.157), macroscopic appearance (P = 0.571) and age of patients (P = 0.438) were analysed and compared between both APC positive and negative adenomas.
CONCLUSION: Most adenomas expressed full-length APC protein, suggesting that protein expression is not a reliable marker for assessment of APC gene mutation. Complete loss of APC protein did not influence morphology, location, or appearance of adenomas, nor was it affected by the patient’s age.
- Citation: Bortlík M, Vítková I, Papežová M, Kohoutová M, Novotný A, Adamec S, Chalupná P, Lukáš M. Deficiency of Adenomatous Polyposis Coli protein in sporadic colorectal adenomas and its associations with clinical phenotype and histology. World J Gastroenterol 2006; 12(24): 3901-3905
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3901
Adenomatous polyposis coli (APC) gene is thought to play a key role in the pathogenesis of colorectal cancer not only in patients with familial adenomatous polyposis syndrome (FAP), but also in the majority of patients with sporadic colorectal neoplasia[1]. About 60%-80% of sporadic carcinomas of the large bowel carry at least one mutation in the APC gene and a similar frequency was reported in colorectal adenomas[2-4]. Hence, a mutation in the APC gene is suspected to be an early event in the process of colorectal carcinogenesis[5].
There are numerous known, functionally important mutations in the APC gene. Almost all of the mutations implicated in the process of the neoplastic conversion lead to a stop codon with subsequent synthesis of a truncated APC protein, which lacks the carboxyl-terminal part[5,6]. Loss of this part of the APC protein results in the absence of almost all binding sites for β-catenin, which, if not bound in a multiprotein complex with APC, translocates into the nucleus, where it promotes the process of malignant transformation[6]. The absence of the full-length and functional APC protein can be detected by immunohistochemistry using the rabbit or mouse polyclonal antibodies[7,8]. Iwamoto et al reported the results of an immunohistochemical analysis, where the frequency of the complete loss of the APC protein was substantially lower in colorectal adenomas than that detected in carcinomas[9]. Another authors also studied the relationship between the APC protein expression and morphological features of colorectal neoplasms, but results are still conflicting[10-12]. Therefore, in our study we investigated the relationship between the loss of the full-length APC protein assessed by immunohistochemistry and some characteristics of colorectal adenomas with special attention to the malignant potential of adenomas.
One hundred and eighteen consecutive patients with endoscopically-removed colorectal adenomas were involved in this study. Patients with known diagnosis of the familial adenomatous polyposis were excluded. In patients with more than one adenoma, only the largest one was used for further assessment. The size of each adenoma was measured, either using the biopsy forceps, having their tips separated in close proximity to the adenoma within the bowel, or after removal of the polyp from the colon. In large, piecemeal, resected tumors, the size was assessed before the adenoma was removed from the bowel. Adenomas arising in the cecum, ascending colon, or transverse colon were defined as right-sided tumors, whereas those arising distally to the splenic flexure were labeled as left-sided adenomas. Appearance of adenomas was defined either as pedunculated, when a visible stalk was present, or sessile, in the absence of a stalk.
All adenomas were evaluated histologically after staining with hematoxylin-eosin by an experienced pathologist (I.V.). An immunohistochemical examination of the APC protein was then performed on all adenomas and evaluated for size, histology and grade of dysplasia, as parameters of the malignant potential. We also included the adenoma’s location, its macroscopic appearance and the patient’s age in the statistical analysis. An informed consent was obtained from all patients and the study was approved by the Ethics Committee of the General Teaching Hospital, Prague.
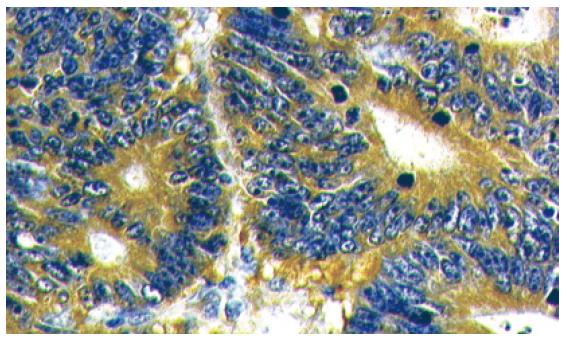
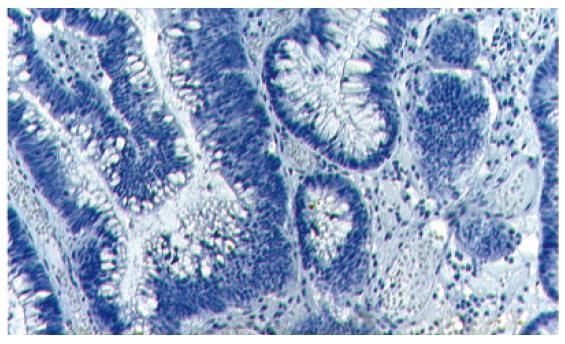
In general, APC immunohistochemistry was performed using the anti-APC antibodies and their dilution as described by Iwamoto et al[9,13]. Paraffin-embedded tissue blocks were cut to obtain sections 4 μm thick, deparaffinized in xylen and rehydrated in a series of graded alcohol + water solutions. Endogenous peroxidase activity was then blocked using a 3% solution of hydrogen peroxide in methanol. Subsequently, tissues were incubated in non-immune serum for 30 min. Next, samples were incubated with anti-APC antibodies (C-terminus, Santa Cruz Biotechnology, Santa Cruz, USA) diluted 1:200 for 60 min. Streptavidin peroxidase (detection kit) with diaminobenzidine (DAB) as a substrate was used for detection at room temperature. Sections were stained with hematoxylin, dehydrated and assembled into the xylen-soluble medium. A definite APC immune reaction in the adjacent normal mucosa of each adenoma served as a control of the normal APC protein reaction.
Normality was tested by Shapiro-Wilk’s W test, as the data did not show a normal distribution, non-parametrical statistical methods were used. The APC status was compared with characteristics of adenomas using the Fisher´s exact test and χ2 (Chi-square) test for categorical variables and the Mann-Whitney test for continuous variables. All tests were bilateral and the significance level was set at 5% for each analysis. We used the statistical software Statistica, version 7, StatSoft, Inc. (2004).
Patients’ demographics and the adenomas’ characteristics are shown in Table 1.
| Patients | n | 118 | |
| Sex | Male/Female | 71 (60%)/47 (40%) | |
| Age (yr) | Mean (range), Median | 63 (25-86), 65 | |
| Adenomas | Size (mm) | Mean (range), Median | 14 (3-55), 6 |
| Location | Left/Right | 83 (70%)/35 (30%) | |
| Histology | Tubular/Tubulo-villous/Villous | 82 (70%)/32 (27%)/4 (3%) | |
| Dysplasia | Low/High-grade | 103 (87%)/15 (13%) | |
| Appearance | Pedunculated/Sessile | 31 (26%)/87 (74%) |
Of 118 adenomas evaluated, 90 (76%) expressed full-length APC protein, whereas 28 (24%) were APC protein-negative (Figures 1 and 2). APC status was then compared with the parameters definining the malignant potential of the adenoma (size, histology and grade of dysplasia) (Table 2).
| Parameter | APC+ | APC- | P | |
| Size (SD) | 13.5 (± 14.2) | 13.8 (±15.5) | 0.3641 | |
| Histology | Tubular | 62 (76%) | 20 (24%) | 0.3272 |
| Tubulovillous | 24 (75%) | 8 (25%) | ||
| Villous | 4 (100%) | 0 (0%) | ||
| Dysplasia | Low-grade | 78 (76%) | 25 (24%) | 0.4943 |
| High-grade | 12 (80%) | 3 (20%) | ||
| Advancement | Advanced | 36 (78%) | 10 (22%) | 0.4143 |
| Non-advanced | 54 (75%) | 18 (25%) | ||
| Location | Left | 66 (80%) | 17 (20%) | 0.1573 |
| Right | 24 (69%) | 11 (31%) | ||
| Appearance | Sessile | 66 (76%) | 21 (24%) | 0.5713 |
| Pedunculated | 24(77%) | 7 (23%) | ||
| Age (yr) | < 41 | 5 (63%) | 3 (37%) | 0.4382 |
| 41-50 | 7 (64%) | 4 (36%) | ||
| 51-60 | 27 (84%) | 5 (16%) | ||
| > 60 | 51 (76%) | 16 (24%) |
The mean size of APC-positive and negative adenomas was 13.5 mm (SD ± 14.2; 95% CI 10.5-16.5) and 13.8 mm (SD ± 15.5; 95% CI 7.8-19.8), respectively. Using the Mann-Whitney test, no statistically significant difference was found between the two groups (P = 0.364). Histological assessment revealed that among 82 tubular adenomas, 20 (24%) were APC-negative, and 62 (76%) were positive for APC by immunostaining. Similarly, of 32 tubulovillous adenomas, eight (25%) were negative, while 24 (75%) were positive for the APC protein. All four villous adenomas expressed the APC protein. No statistically significant relationship was found between the histology and the APC status of adenomas (χ2 test, P = 0.327). Majority of adenomas harboured low-grade dysplasia (n = 103). Twenty five (24%) of them were APC-negative, while the remaining 78 (76%) were positive. Three (20%) adenomas with high-grade dysplasia did not show APC positivity, whereas 12 (80%) were positive. Again, no significant relationship between the APC presence/absence and the grade of dysplasia was observed in our group (Fisher`s exact test, P = 0.494).
We identified a substantial number of advanced adenomas (n = 46, 39% of entire group). Of them, ten (22%) were APC-negative. Similar results were obtained among non-advanced adenomas, where 18 out of 72 (25%) did not express the APC protein. Using the Fisher’s exact test, no significant difference was found between the APC groups (P = 0.414).
Further assessment was performed to look for a relationship between the APC status and the location of adenomas, their macroscopic appearance and the patient’s age (Table 2). More than 2/3 of all adenomas were located distally to the splenic flexure. Seventeen left-sided adenomas (20%) were APC-negative, compared with 11 (31%) APC-negative right-sided tumors (Fisher`s exact test, P = 0.157). Comparison between the APC status and the macroscopic appearance of the adenomas revealed 21 (24%) APC-negative, sessile adenomas. Similarly, seven (23%) APC-negative, pedunculated polyps were observed (Fisher`s exact test, P = 0.571). To evaluate the relationship between the age and APC immunoreactivity, patients were divided into groups according to their age: < 41, 41-50, 51-60, and > 60 years of age. Corresponding numbers of APC-negative adenomas were 3 (37%), 4 (36%), 5 (16%), and 16 (24%), respectively. No significant difference was found among the groups (χ2 test, P = 0.438).
We attempted to verify the hypothesis that the absence of the full-length APC protein resulting from a bi-allelic mutation of the APC gene (or a mutation in one allele and the loss of the other) might be associated with a higher degree of malignant potential or with an advanced type of adenoma. We found a complete loss of the APC immunoreactivity in a relatively small proportion of adenomas (24%), whereas more than 3/4 of them displayed either complete, or partial positive results in APC immunostaining. This result is, to some degree, inconsistent with the hypothesis that complete inactivation of both alleles of the APC gene occurs early during the process of colorectal tumorigenesis. These data were derived from studies demonstrating a similar frequency of APC mutation, about 60%-80%, in both carcinomas and adenomas[2,3-5]. Contrary to this, our results might indicate that the large number of adenomas contain the mutation of only one allele of the APC gene. Whereas 45%-60% of all sporadic adenomas and 60%-80% of carcinomas harbour one inactivated allele, a bi-allelic defect could be found in approximately 30% of adenomas[5,8]. One possible explanation for this finding is that the carcinogenesis of the sporadic colorectal tumors differs from that in patients with familial adenomatous polyposis syndrome, where the vast majority of adenomas harbour a bi-allelic mutation of the APC gene[14,15].
However, it is necessary to emphasize that immunohistochemistry detects the presence of the full-length APC protein instead of the actual mutation of the gene. It is the current assumption that almost all of the APC gene’s mutations result in the production of a truncated protein that has lost its tumor suppressor function[6]. The absence of the full-length APC protein thus indicates a bi-allelic loss of the gene, caused by two mutations or by the combination of one allele’s mutation with another type of genetic or epigenetic event (allelic loss or promoter hypermethylation). No clear relationship between the APC protein expression and adenomas’ morphology was observed in our study. While some authors found higher frequency of the APC gene mutations in tubulovillous and villous adenomas[16], others refuted this observation[9]. The lack of significant difference between adenomas with different malignant potential is contrasted by an abrupt increase in the APC protein loss in carcinomas, which harbour a complete (bi-allelic) APC defect much more frequently. Such results were published by Iwamoto et al, who found completely negative response for APC immunostaining in 83% of carcinomas, but in only 29% of adenomas[7]. Moreover, even when the exact proportion of APC gene mutations or other epigenetic changes were assessed, results were different for adenomas compared with carcinomas[5,8]. On the other hand, Chen et al demonstrated positivity of the APC protein detected immunohistochemically and by western blot analysis in the majority of colon cancers[17]. In this study, the APC protein levels were not different among tissues with unchanged or mutated APC gene. Even hypermethylation of the APC gene, which seems to be a relatively frequent mechanism of APC silencing[18], did not result in APC protein negativity.
Sporadic adenomas may grow with only one allele inactivated[19] as a result of, for example, dominant-negative effect of APC protein homodimers formed by a truncated protein together with the full-length APC protein[20]. These homodimers prevent normal function of APC protein but not its immunoreactivity. This theory is supported by the finding of the nuclear, so that abnormal accumulation of β-catenin in adenomas with a definite positive immunohistochemical result of the APC protein[7]. Therefore, the presence of the APC immunostaining does not necessarily mean the presence of functioning APC protein. Furthermore, mutations of APC may have direct oncogenic effect. Tighe et al suggested that the initial APC mutation acts as a “double whammy”, destabilising the genome and setting the stage for deregulated proliferation upon loss of the second APC allele[21].
A key question is whether the presence, or absence, of the full-length APC protein affects the prognosis of adenomas as to their future malignant transformation. Theoretically, the lack of the APC protein should stimulate adenomas to grow and undergo malignant transformation. While some reports, including our results, showed no different morphology of APC-negative and positive adenomas[7,9], others found that the prevalence of APC mutation continuously increased in the transformation from normal mucosa to carcinoma[8], or that the frequency of APC mutations in tubulovillous or villous adenomas was higher than in tubular adenomas[10]. Among our adenoma specimens, a lack of APC protein was observed even in some polyps smaller than 5 mm, which are generally thought to be of minimal clinical importance. On the other hand, several investigators have observed that there are many other genes involved in colorectal carcinogenesis and that a significant number of adenomas, as well as carcinomas, do not harbor any APC gene mutations at all[8,16,22]. It should also be noted that colorectal cancers are genetically heterogeneous, multiple genes and mutation may be involved in the tumorigenesis simultaneously as well as the key mechanism may be different in different parts of the colon.
In conclusion, we have not observed a relationship between the presence, or absence, of the full-length APC protein and histology as well as clinical phenotype of sporadic colorectal adenomas. Moreover, only a small part of adenomas did not express the APC protein as assessed by immunohistochemistry. Both these results may indicate that adenomas either harbour genetically heterogenous background, or that one allele remains unchanged and still allows production of normal, full-length APC protein. Hence, immunohistochemistry seems to be an unreliable marker for assessment of APC gene changes in colorectal adenomas.
Adam Svobodnik for his cooperation during the statistical analysis, Jelena Vavrova and Peter Laszlo Lakatos for their kind help with the manuscript preparation.
S- Editor Pan BR L- Editor Ma JY E- Editor Zhang Y
| 1. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 7984] [Article Influence: 228.1] [Reference Citation Analysis (1)] |
| 2. | Nagase H, Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat. 1993;2:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 259] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol Cancer. 2003;2:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK, Paraskeva C, Petersen GM, Hamilton SR, Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993;90:2846-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 294] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1300] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 621] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Midgley CA, White S, Howitt R, Save V, Dunlop MG, Hall PA, Lane DP, Wyllie AH, Bubb VJ. APC expression in normal human tissues. J Pathol. 1997;181:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Losi L, Di Gregorio C, Pedroni M, Ponti G, Roncucci L, Scarselli A, Genuardi M, Baglioni S, Marino M, Rossi G. Molecular genetic alterations and clinical features in early-onset colorectal carcinomas and their role for the recognition of hereditary cancer syndromes. Am J Gastroenterol. 2005;100:2280-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Iwamoto M, Ahnen DJ, Franklin WA, Maltzman TH. Expression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis. 2000;21:1935-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Kim JC, Koo KH, Roh SA, Cho YK, Kim HC, Yu CS, Kim HJ, Kim JS, Cho MK. Genetic and epigenetic changes in the APC gene in sporadic colorectal carcinoma with synchronous adenoma. Int J Colorectal Dis. 2003;18:203-209. [PubMed] |
| 11. | Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54:3011-3020. [PubMed] |
| 12. | Mulkens J, Poncin J, Arends JW, De Goeij AF. APC mutations in human colorectal adenomas: analysis of the mutation cluster region with temperature gradient gel electrophoresis and clinicopathological features. J Pathol. 1998;185:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Iwamoto M, Hoffenberg EJ, Carethers JM, Doctolero R, Tajima A, Sugano K, Franklin WA, Ahnen DJ. Nuclear accumulation of beta-catenin occurs commonly in the epithelial cells of juvenile polyps. Pediatr Res. 2005;57:4-9; discussion 1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, Frayling I, Efstathiou J, Pack K, Payne S. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's 'two-hit' hypothesis. Nat Med. 1999;5:1071-1075. |
| 15. | Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | De Benedetti L, Sciallero S, Gismondi V, James R, Bafico A, Biticchi R, Masetti E, Bonelli L, Heouaine A, Picasso M. Association of APC gene mutations and histological characteristics of colorectal adenomas. Cancer Res. 1994;54:3553-3556. [PubMed] |
| 17. | Chen J, Röcken C, Lofton-Day C, Schulz HU, Müller O, Kutzner N, Malfertheiner P, Ebert MP. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis. 2005;26:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366-4371. [PubMed] |
| 19. | Pretlow TP, Edelmann W, Kucherlapati R, Pretlow TG, Augenlicht LH. Spontaneous aberrant crypt foci in Apc1638N mice with a mutant Apc allele. Am J Pathol. 2003;163:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Bodmer W, Bishop T, Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994;6:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci. 2004;117:6339-6353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:9433-9438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |