Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3814
Revised: February 9, 2006
Accepted: February 18, 2006
Published online: June 28, 2006
AIM: To demonstrate an inexpensive method for typing gastric cancer related single nucleotide polymorphisms (SNPs) using whole blood or paper-dried blood as starting materials.
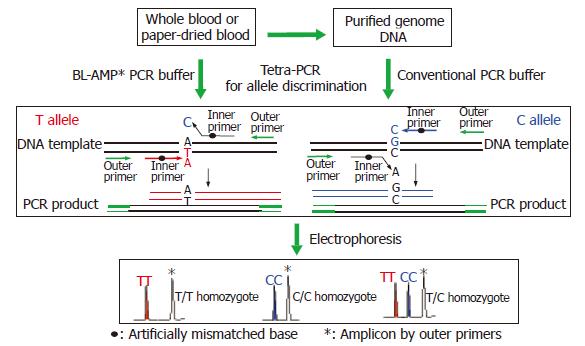
METHODS: PCR amplification is directly carried out from the whole blood or paper-dried blood sample without any DNA extraction step. Before PCR, a blood sample, four primers, and all of biological reagents necessary for PCR were added at a time; After PCR, the amplified products were directly separated by slab gel electrophoresis or microchip CE without any purification. SNP typing was performed by tetra-primer PCR with two inner primers specific to each allele and two outer primers defining the length of allele-specific amplicons. Genotypes were directly discriminated by the size of amplicons specific to each allele, thereby avoiding any post-PCR process.
RESULTS: Using a special PCR buffer, inhibitory substances in blood (including the anticoagulant in blood) and filter paper were effectively suppressed; a “true” single-tube-genotyping is thus realized. We successfully determined genotypes IL-1B-511 and IL-1B-31 polymorphisms at the gene IL-1B by using whole-blood and paper-dried blood samples as starting materials respectively. The method is so sensitive that 0.5-1.0 μL of blood sample is enough to give a satisfactory typing results. The genotyping results were confirmed by RFLP-PCR using purified genome DNA, indicating that amplification specificity was not affected by inhibitory components (including coagulants) in blood or filter paper.
CONCLUSION: Compared with SNP typing methods based on purified DNA, the proposed method is labor-saving, simple, inexpensive, and less cross-contaminated. It is promising to use this method to type other SNPs.
- Citation: Huang H, Bu Y, Zhou GH. Single-tube-genotyping of gastric cancer related SNPs by directly using whole blood and paper-dried blood as starting materials. World J Gastroenterol 2006; 12(24): 3814-3820
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3814.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3814
Gastric cancer is a complex disease with a high mortality rate[1-3], but the recent success of the Human Genome Project[4] as well as the identification of vast numbers of single nucleotide polymorphisms (SNPs)[5] enables us to prevent or alleviate the disease by detecting the potential disease-susceptibility alleles and diallelic markers. It was reported that cytokine IL-1B, a potent proinflammatory cytokine that is also a powerful inhibitor of gastric acid secretion, is up-regulated by H pylori infection and is important in initiating and amplifying the inflammatory response to this infection[6]. Genetic analysis from different race’s population[6-12] showed that the IL-1B-511 and -31 polymorphisms in the promoter region are associated with the development of gastric cancer. Currently, several different IL-1B genotyping techniques have been described; these include restriction fragment length polymorphisms (RFLP)[7,12-14] analysis and pyrosequencing. Although RFLP is very simple and can be performed without any expensive instrument, post-PCR process and a particular restriction endonuclease are required. Pyrosequencing is a newly developed typing method based on a real-time DNA sequencing[15,16], but an expensive pyrosequencer is needed for the detection.
Up-to-now, various typing techniques based on different principles, e.g., sequence-specific hybridization[17,18], allele-specific extension[19,20] or ligation[21,22], and structure specific cleavage[23,24], have been developed. However, almost all of these methods require purified genome DNA as the starting material for PCR. The genome DNA extraction is costly and time-consuming. More over, DNA isolation process causes the risk of sample contamination with foreign DNAs. Since a huge number of SNPs should be typed through multiple regions of interest for clarifying the disease-susceptibility SNPs, a simple, inexpensive and labor-saving genotyping method without the DNA extraction procedure is preferable. Here we propose a single-tube genotyping method by combining whole blood PCR (WB-PCR) and tetra-PCR[25,26]. Neither a post-PCR step nor an expensive instrument is required. The type of SNP could be determined by the length of the amplicons from PCR with whole blood or paper-dried blood in a single tube containing four primers: two inner primers specific to each allele and two outer primers defining the length of allele-specific amplicons. To reduce the sample consumption and to increase the typing speed, microchip electrophoresis[27] was used for the detection. As shown in Figure 1, the procedure is very simple, and all of the reagents necessary for PCR as well as blood sample were added in the tube at a time before PCR. Therefore, the aim of the present study is to demonstrate an inexpensive method for typing gastric cancer related SNPs using whole blood or paper-dried blood as starting materials.
Taq DNA polymerase and ethidium bromide were from Promega (Madison, USA). Agarose gel was from Oxoid Limited (Basingstoke Hampshire, England), and dNTP was from Genebase Gene-Tech (Shanghai, China). All solutions were prepared in deionized and sterilized water. DNA 1000 ladder, DNA 1000 Marker15/1500 bp, DNA Dye, and DNA Gel Matrix were purchased from Agilent technologies. The other reagents used in the experiment are analytical pure grade or molecule biology grade. PCR buffer BL-AMP™ was from Bailong Gene Company (Nanjing, China).
Human blood samples were obtained from 40 patients with clinical confirmation and informed consent. Whole-blood samples were venously collected and treated with dipotassium EDTA anticoagulant. The blood sample for condition test was from a volunteer in our laboratory, and directly treated with dipotassium EDTA, sodium citrate, and sodium heparinate respectively. All of the whole blood samples were stored at + 4°C before use. Paper-dried blood was prepared by dropping a small amount of anticoagulant blood sample on a piece of sterile filter paper, followed by air dry at room temperature, and stored in vacuum desiccator at room temperature before use.
Genomic DNA was extracted from blood samples using standard procedures with phenol-chloroform extraction method to separate proteins from nucleic acids[28]. DNA isolation was carried out by suspending whole blood in equal volume of lyses buffer (10 mmol/L Tris-HCl, pH 7.6, 50 mmol/L MgCl2, 320 mmol/L sucrose and 10 g/L Triton-100). After mixing and centrifugation (2 min at micro-centrifuge), the supernatant fluid was removed. This isolation procedure was performed twice. Then the pellet was dissolved with TES buffer (15 mmol/L Tris-HCl, 15 mmol/L EDTA, 15 mmol/L NaCl, 5 mol/L NaClO4, and 100 g/L SDS; pH 8.0 ), and incubated at 37°C for 15 min and at 65°C for 20 min. DNA was extracted twice with 1 volume of phenol-chloroform-isoamyl alcohol (25:24:1). Nucleic acids remained in the aqueous phase; cellular proteins were separated into the organic phase or lay at the phase interface. After centrifugation, the organic phase was discarded and the aqueous phase was precipitated with absolute ethanol. The pellet was washed with 700 mL/L ethanol, dried in the still air and suspended in Tris-EDTA buffer (10 mmol/L Tris base, 1 mmol/L EDTA; pH 8.0). The DNA was quantified spectrophotometrically at 260 nm, and then stored at 4°C before use.
The nucleotide sequences for the IL-1B gene are available from Genbank (http://www.ncbi.nlm.nih.gov/), and the accession number is AY137079. IL-1B polymorphic sites in the promoter region at position -31 and -511 were genotyped. For typing one SNP, four primers are necessary: two allele-specific primers (S1-1, S1-2 or S2-1, S2-2; Table 1) and two outer primers (P1-1, P1-2 or P2-1, P2-2; Table 1). The sequences of all the primers were listed in the Table 1. A mismatched base was introduced into the third position upstream from 3’ terminal of the allele-specific primer to decrease the probability of spurious extension reaction. Genotypes of IL-1B-31 were determined by a 258-bp PCR fragment (specific to IL-1B-31T) with primers P1-1 and S1-1, and a 162-bp PCR fragment (specific to IL-1B-31C) with primers P1-2 and S1-2. Genotypes of IL-1B-511 were determined by a 356-bp fragment (specific to IL-1B-511C) with primers P2-1 and S2-1, and a 226-bp fragment (specific to IL-1B-511T) with primers P2-2 and S2-2. The software of Primer Premier Version 5.0 (http://www.premierbiosoft.com/) was used to calculate the melting point and to analyze the risk of primer-dimer formation and secondary structures. All the oligonucleotides were synthesized and purified by Invitrogen Biotechnology Co., Ltd (Shanghai, China).
| Polymorphism | Primer sequences |
| IL-1B-31 | P1-1 5’- tcaactgcacaacgattgtca -3’ |
| P1-2 5’- tcccttagcacctagttgtaa -3’ | |
| S1-1 5’- ttctccctcgctgtttttGta -3’ | |
| S1-2 5’- ctacttctgcttttgaaaCcc -3’ | |
| IL-1B-511 | P2-1 5’-ggaagggcaaggagtagcaaac-3’ |
| P2-2 5’-ggcagagctcatctggcattga-3’ | |
| S2-1 5’-ctgcaattgacagagagctGcc-3’ | |
| S2-2 5’-ttgggtgctgttctctgccAca-3’ |
Purified DNA template was amplified in a final volume of 15 μL containing 0.2 mmol/L dNTP mixture, 1.67 μmol/L of each of four primers (IL-1B-31), or 2.67 μmol/L of each outer-primer and 0.67 μmol/L of each inner-primer (IL-1B-511), 1 U of Promega Taq DNA polymerase, 10 × Buffer, and 1.5 mmol/L MgCl2. Thermocycling was carried out on PTC-225 Peltier Thermal Cycler (MJ RESEARCH, INC.) at an initial denaturation at 96°C for 3 min, then 35 cycles of 94°C for 30 s, 52°C (IL-1B-31) or 58°C (IL-1B-511) for 1 min, and 72°C for 1 min, followed by 72°C for 7 min. Whole blood or paper-dried blood sample was amplified in a 20 μL PCR mixture containing BL-AMP™ buffer instead of the conventional 10 × PCR buffer, 0.2 mmol/L dNTP mixture, 1.5 mmol/L MgCl2, 2 U of Promega Taq DNA polymerase, and 1.5 μmol/L of each of four primers (IL-1B-31), or 3 μmol/L of each outer-primer and 0.75 μmol/L of each inner-primer (IL-1B-511). Following the assembly of the PCR mixture, anticoagulant-treated whole blood or filter paper-dried blood was added (commonly 1 μL of blood per 50 μL of PCR mixture) into the bottom of the PCR tube without any agitation. Thermocycling was carried out at an initial denaturation at 96°C for 3 min, then 40 cycles of 94°C for 30 s, 52°C (IL-1B-31) or 58°C (IL-1B-511) for 1 min, and 72°C for 1 min, followed by 72°C for 7 min.
PCR products were separated by either gel electrophoresis or microchip electrophoresis. The gel electrophoresis was performed on a 20 g/L agarose gel stained with 0.005 g/L EtBr in 1 × TAE buffer at 100 V for 14 min. Gel images were captured on a Bio Imaging System (Syngene, UK). Microchip electrophoresis was carried out on Agilent 2100 bioanalyzer (Agilent technologies, German) according to the manufacture’s instructions.
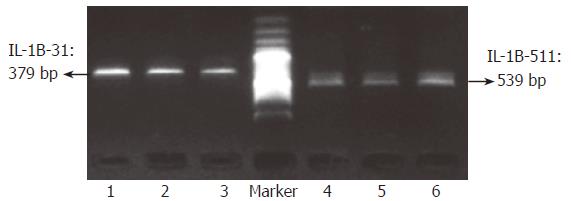
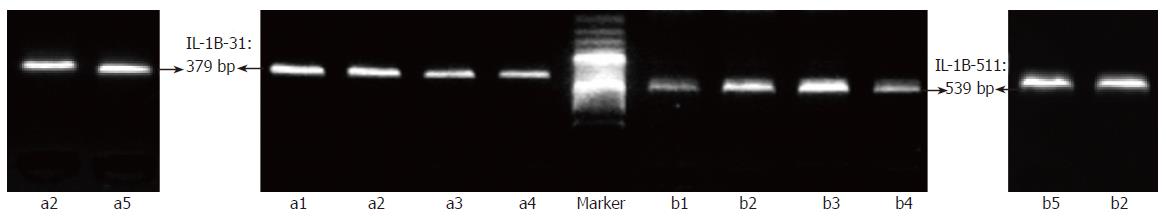
To evaluate whether or not target sequences in whole blood or paper-dried blood could be amplified using the “BL-AMP™” buffer, PCR with two sets of primers (P1-1 and P1-2, and P2-1 and P2-2; Table 1), which were used as outer primers for typing IL-1B-31 and -511 polymorphisms, were carried out. Except for PCR buffer, all PCR conditions including Taq polymerase, concentration of Mg2+ and dNTPs and thermal cycling conditions, are the same as that used in conventional PCR. As shown in Figure 2, the fragments with the expected length of 379 bp and 539 bp were successfully amplified from whole blood with the anticoagulant of dipotassium EDTA, sodium citrate, or sodium heparinate respectively. To check whether paper constitutes suppress PCR or not, a piece of filter paper with 1 mm2 was added into 50 μL of PCR reaction mixture containing purified genome DNA; and the results showed that “BL-AMP™” buffer could block the PCR inhibition by the chemicals present in filter paper (data not shown). Direct PCR with two sets of primers (P1-1 and P1-2, and P2-1 and P2-2; Table 1) using 0.5, 1, 2 and 4 μL of EDTA-blood dried on filter paper as starting materials were carried out to evaluate the sensitivity (Figure 3); and PCR with 1 μL of fresh blood without an anticoagulant was performed in parallel for the comparison. Successful PCRs were obtained for all samples, and 1 μL of paper-dried blood gave an optimum sensitivity.
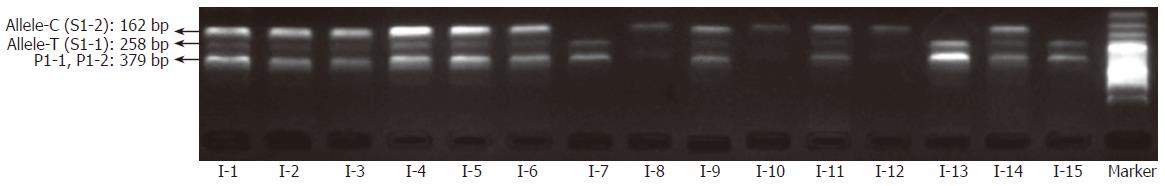
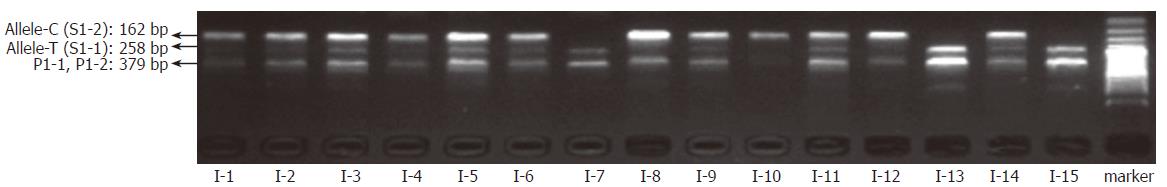
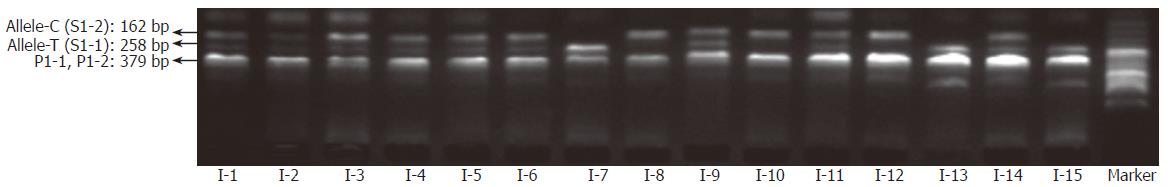
By coupling the “BL-AMP™” buffer with tetra-PCR, genotyping of IL-1B-31 and -511 polymorphisms was carried out by using whole blood (with dipotassium EDTA anticoagulant) and dried blood (on a piece of sterile common filter paper) as the starting material respectively. Samples from fifteen different individuals were employed for the evaluation. Amplicons were separated by slab gel electrophoresis that is a simple and inexpensive way in a regular lab. As shown in Figures 4 and 5, it is very easy to get unambiguous SNP types from electrophoretic patterns by using whole-blood samples or paper-dried blood samples as PCR starting materials. As the amplification efficiency of two allele-specific amplicons is different, the concentration ratios of two allele-specific primers were optimized as 8:1 (S1-1:S1-2) to get a relatively equal band-intensities (Figure 6). The bands of 162 bp and 258 bp represent allele C and T respectively. The band of 379 bp was the amplicon by two outer primers P1 and P2; this band was used to be a positive control for checking the sample quality. For a heterozygote sample, both 162- and 258-bp fragments were co-amplified, while either 162- or 258-bp fragment was amplified if the sample was of a homozygote. All of three genotypes were unambiguously determined, indicating that the newly developed method is highly specific.
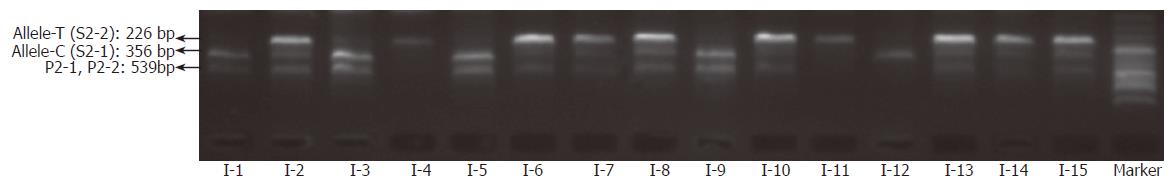
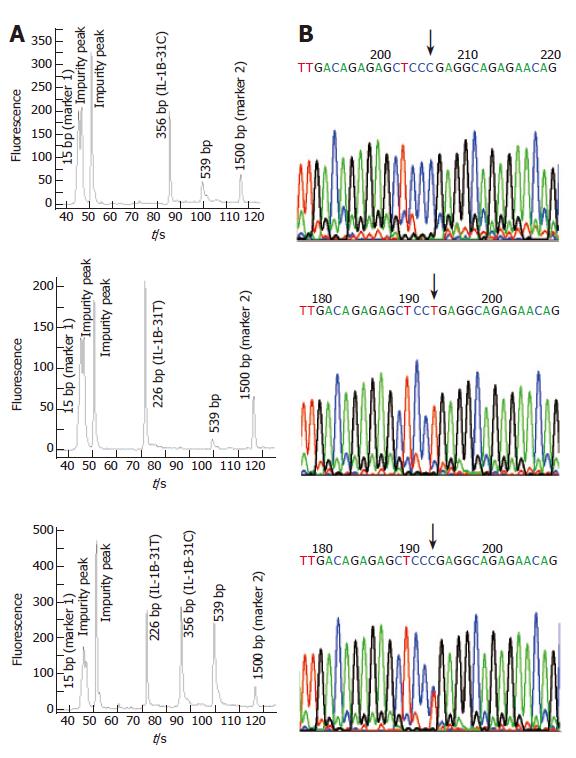
To test the effect of inhibitors in blood or paper tissues on the specificity, genotyping was also performed on the genome DNAs extracted from the same blood samples as those in Figure 4; the results (Figure 7) were in complete agreement with those obtained with whole blood or paper-dried blood samples. Therefore the presence of the inhibitors did not interfere with the specificity of genotyping. By using the same condition, polymorphism of IL-1B-511 was typed directly from fifteen blood samples as shown in Figure 8. The allele-specific PCR fragments are marked by allele-C and allele-T, which correspond to the amplicons with the size of 226 bp and 356 bp respectively. A 539-bp amplicon was from two outer primers (P2-1 and P2-2). The unambiguous typing results of all tested samples were obtained. Also the genotyping results of IL-1B-511 polymorphism from three different starting materials (whole blood, paper-dried blood and purified DNA) are completely consistent (data not shown).
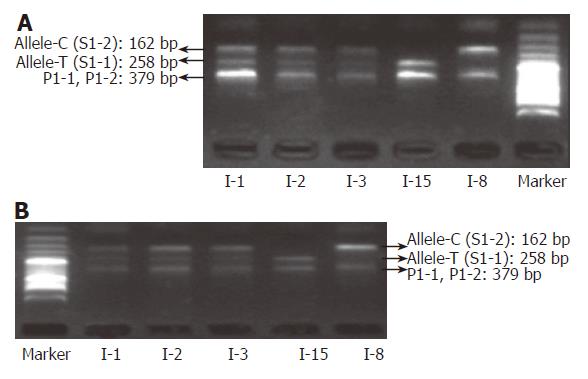
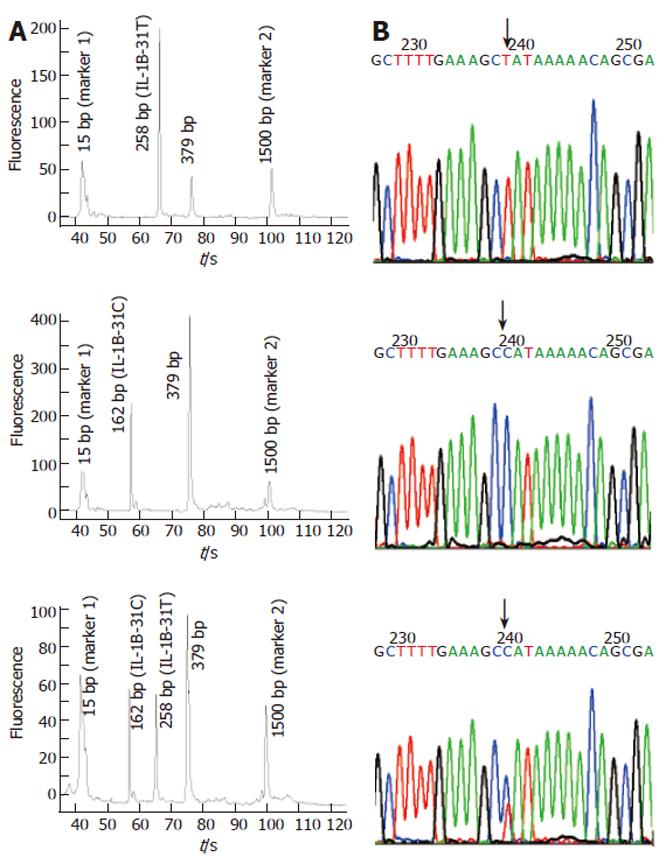
Compared with slab gel electrophoresis, microchip electrophoresis is time-saving, labor-saving, and extremely sensitive. As the separation is in the microchannel, a small amount of DNA sample is enough for getting a reliable result; the typing cost can thus be further decreased. In this report, Agilent 2100 bioanalyzer was employed to analyze the amplified products of the above fifteen blood samples. With the DNA markers of different sizes, the length of each amplicon was identified automatically. We found that all blood-based tetra-PCR amplicons gave the expected sizes. Figures 9A and 10A show the typing results of three typical genotypes (two kinds of homozygotes and a heterozygote) of IL-1B-31 and IL-1B-511 respectively.
To evaluate the accuracy of SNP typing, five representative individuals including heterozygotes and both homozygotes were selected and genotyped again by Sanger’s sequencing method using purified genomic DNA. The sequencing-based typing results of the five individuals completely coincided with those by the single-tube tetra-PCR. For the comparison, sequencing spectra of the samples used in Figures 9A and 10A were listed as Figure 9B and 10B respectively, indicating that it is very accurate to apply the newly developed single-tube method for genotyping IL-1B-31 and -511 polymorphisms.
To test the robustness of the method, we analyzed three blood samples with typical genotypes for ten times at ten different days. The consistent typing results at both IL-1B-31 and IL-1B-511 polymorphisms were obtained, indicating that the proposed method is reproducible and reliable. In addition to the blood samples from 15 individuals, we also typed another 25 whole-blood samples by using the proposed single-tube-genotyping method; and unambiguous typing results of both IL-1B-31 and IL-1B-511 polymorphisms were obtained (data not shown).
Tetra-PCR is very simple and time-saving[25,26]; but it is not a true single-tube genotyping method as DNA isolation step was still required. Since many substances existing in blood, e.g. polysaccharides, proteins, lipids and haemoglobin, suppress PCR[29], it is very difficult to escape the DNA extraction step and directly use whole blood for PCR amplification. Moreover, anticoagulants, such as EDTA or heparin, which are commonly added to prevent blood samples from the clotting, may interfere with PCR amplification. To effectively suppress the inhibitory substances including the anticoagulant in blood, a novel PCR buffer named “BL-AMP™” was developed.
By coupling tetra-PCR amplification with this buffer, a single-tube-genotyping method using the whole blood and paper-dried blood as the starting material was realized. To type each allele, four primers (two outer primers and two inner primers) were used for amplifying two allele-specific fragments. Two inner primers complementary to sense and non-sense strands respectively were used to discriminate the allele. As one base differing at the 3’-ends of each inner primer is not enough to perfectly switch the allele-specific extension reaction, an artificial mismatch base was introduced into the third base from the 3’-ends. Two outer primers define the length of two allele-specific amplicons. After PCR, amplicons were separated by electrophoresis, e.g., slab gel electrophoresis or microchip electrophoresis. The SNP type is discriminated by the pattern in the electrophoretogram; two bands mean a heterozygote and one band means a homozygote. The length of the band determines the genotype of the homozygote. Unlike Taqman, molecular beacon, or minisequencing, the newly proposed method does not require labeling, and less sample manipulation than RFLP is needed. As the success in avoiding DNA isolation, the genotyping is a true single-tube format by using “BL-AMP™” buffer for PCR.
From the results of our study, it is demonstrated that PCR amplification using “BL-AMP™” buffer is independent of the type of anticoagulant in blood. In terms of transportation and storage of blood samples, it is very convenient to use filter paper as a carrier because no refrigeration is required for paper-dried blood; this is thus very useful to collect blood samples in remote areas. In addition, no significant difference in PCR efficiency between 1-μL fresh blood and dried blood equivalent to 1-μL fresh blood was observed. Compared with conventional PCR using purified DNA, a reddish color in the final PCR products was observed; however, this would not interfere with the detection of PCR products via electrophoresis. Consequently whole blood or paper-dried blood other than purified genome DNA can be employed for the starting materials of PCR.
Although no clean-up process was used after whole-blood PCR, direct microchip electrophoresis of raw PCR products gave a steady baseline. It is straightforward to discriminate genotypes with peaks in the electrophoreic spectra. Based on the peak assignment, genotypes can be accurately determined even if unspecific amplicons occurred in the spectra, so that it becomes easier to design primers for SNP typing by tetra-PCR. On the other hand, it is not necessary to optimize the primer concentration to get an even amplification as the Agilent 2100 bioanalyzer can assign the size to peaks with lower intensities.
In summary, we have successfully proposed a simple, labor-saving, inexpensive and rapid genotyping method that directly uses whole blood or paper-dried-blood as starting materials for SNP typing in a single tube. The successful genotyping of the IL-1B-31 and -511 polymorphisms demonstrated the reliability of the single-tube genotyping using whole blood or paper-dried blood as the starting material. As the DNA extraction step is taken out, the possibility of sample contamination is significantly decreased. By coupling CE-chip for the readout, an automatic and high-throughput genotyping could be achieved. In the post-genome era, a simple and inexpensive method for diagnostic analysis is highly demanded, so we believe that the whole-blood PCR should be very useful for genetic diagnosis in biologic laboratories.
S- Editor Pan BR L- Editor Zhao JB E- Editor Bi L
| 1. | Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol. 2005;11:3189-3196. [PubMed] |
| 2. | Zhang C, Liu ZK. Gene therapy for gastric cancer: a review. World J Gastroenterol. 2003;9:2390-2394. [PubMed] |
| 3. | Echem R. Gastric cancer is a major cause of cancer death. Niger J Med. 2003;12:175-176. [PubMed] |
| 4. | McPherson JD, Marra M, Hillier L, Waterston RH, Chinwalla A, Wallis J, Sekhon M, Wylie K, Mardis ER, Wilson RK, Fulton R, Kucaba TA, Wagner-McPherson C, Barbazuk WB, Gregory SG, Humphray SJ, French L, Evans RS, Bethel G, Whittaker A, Holden JL, McCann OT, Dunham A, Soderlund C, Scott CE, Bentley DR, Schuler G, Chen HC, Jang W, Green ED, Idol JR, Maduro VV, Montgomery KT, Lee E, Miller A, Emerling S, Kucherlapati, Gibbs R, Scherer S, Gorrell JH, Sodergren E, Clerc-Blankenburg K, Tabor P, Naylor S, Garcia D, de Jong PJ, Catanese JJ, Nowak N, Osoegawa K, Qin S, Rowen L, Madan A, Dors M, Hood L, Trask B, Friedman C, Massa H, Cheung VG, Kirsch IR, Reid T, Yonescu R, Weissenbach J, Bruls T, Heilig R, Branscomb E, Olsen A, Doggett N, Cheng JF, Hawkins T, Myers RM, Shang J, Ramirez L, Schmutz J, Velasquez O, Dixon K, Stone NE, Cox DR, Haussler D, Kent WJ, Furey T, Rogic S, Kennedy S, Jones S, Rosenthal A, Wen G, Schilhabel M, Gloeckner G, Nyakatura G, Siebert R, Schlegelberger B, Korenberg J, Chen XN, Fujiyama A, Hattori M, Toyoda A, Yada T, Park HS, Sakaki Y, Shimizu N, Asakawa S, Kawasaki K, Sasaki T, Shintani A, Shimizu A, Shibuya K, Kudoh J, Minoshima S, Ramser J, Seranski P, Hoff C, Poustka A, Reinhardt R, Lehrach H; International Human Genome Mapping Consortium. A physical map of the human genome. Nature. 2001;409:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 554] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2094] [Cited by in RCA: 1891] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 6. | zur Hausen A, Crusius JB, Murillo LS, Alizadeh BZ, Morré SA, Meijer CJ, van den Brule AJ, Peña AS. IL-1B promoter polymorphism and Epstein-Barr virus in Dutch patients with gastric carcinoma. Int J Cancer. 2003;107:866-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Lee SG, Kim B, Yook JH, Oh ST, Lee I, Song K. TNF/LTA polymorphisms and risk for gastric cancer/duodenal ulcer in the Korean population. Cytokine. 2004;28:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lee SG, Kim B, Choi W, Lee I, Choi J, Song K. Lack of association between pro-inflammatory genotypes of the interleukin-1 (IL-1B -31 C/+ and IL-1RN *2/*2) and gastric cancer/duodenal ulcer in Korean population. Cytokine. 2003;21:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Perri F, Piepoli A, Bonvicini C, Gentile A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR, Ricciardiello L. Cytokine gene polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. Cytokine. 2005;30:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Tatemichi M, Sawa T, Gilibert I, Tazawa H, Katoh T, Ohshima H. Increased risk of intestinal type of gastric adenocarcinoma in Japanese women associated with long forms of CCTTT pentanucleotide repeat in the inducible nitric oxide synthase promoter. Cancer Lett. 2005;217:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Yang J, Hu Z, Xu Y, Shen J, Niu J, Hu X, Guo J, Wei Q, Wang X, Shen H. Interleukin-1B gene promoter variants are associated with an increased risk of gastric cancer in a Chinese population. Cancer Lett. 2004;215:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Zambon CF, Basso D, Navaglia F, Belluco C, Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Chang YW, Jang JY, Kim NH, Lee JW, Lee HJ, Jung WW, Dong SH, Kim HJ, Kim BH, Lee JI. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1beta cytokine in Korean patients with gastric cancer. Int J Cancer. 2005;114:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta. 2006;363:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001;11:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 428] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Bourgeois S, Labuda D. Dynamic allele-specific oligonucleotide hybridization on solid support. Anal Biochem. 2004;324:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Heymann GA, Hoppe B, Nagy M, Schoenemann C, Kiesewetter H, Salama A. B*4440: a novel HLA-B allele identified by sequence-specific oligonucleotide hybridization and sequence-specific amplification. Tissue Antigens. 2005;65:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Zhou GH, Shirakura H, Kamahori M, Okano K, Nagai K, Kambara H. A gel-free SNP genotyping method: bioluminometric assay coupled with modified primer extension reactions (BAMPER) directly from double-stranded PCR products. Hum Mutat. 2004;24:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Hultin E, Käller M, Ahmadian A, Lundeberg J. Competitive enzymatic reaction to control allele-specific extensions. Nucleic Acids Res. 2005;33:e48. [PubMed] |
| 21. | Ficht S, Dose C, Seitz O. As fast and selective as enzymatic ligations: unpaired nucleobases increase the selectivity of DNA-controlled native chemical PNA ligation. Chembiochem. 2005;6:2098-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Bakht S, Qi X. Ligation-mediated rolling-circle amplification-based approaches to single nucleotide polymorphism detection. Expert Rev Mol Diagn. 2005;5:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Olivier M. The Invader assay for SNP genotyping. Mutat Res. 2005;573:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Rao KV, Stevens PW, Hall JG, Lyamichev V, Neri BP, Kelso DM. Genotyping single nucleotide polymorphisms directly from genomic DNA by invasive cleavage reaction on microspheres. Nucleic Acids Res. 2003;31:e66. [PubMed] |
| 25. | Chiapparino E, Lee D, Donini P. Genotyping single nucleotide polymorphisms in barley by tetra-primer ARMS-PCR. Genome. 2004;47:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:E88-E88. [PubMed] |
| 27. | Zhang L, Dang F, Baba Y. Microchip electrophoresis-based separation of DNA. J Pharm Biomed Anal. 2003;30:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Köchl S, Niederstätter H, Parson W. DNA extraction and quantitation of forensic samples using the phenol-chloroform method and real-time PCR. Methods Mol Biol. 2005;297:13-30. [PubMed] |