Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3770
Revised: January 28, 2006
Accepted: February 20, 2006
Published online: June 21, 2006
AIM: To purify and characterize α-L-fucosidase from human liver cancer tissue and to detect the localization of α-L-fucosidase in tumor tissue.
METHODS: Cation exchange chromatography on CM-52 and ultrafiltration were used to separate α-L-fucosidase (AFU) from crude extract of liver cancer tissue. 4-methylumbelliferyl-α-L-fucopyranoside was used as a fluorescent substrate to quantify the purified AFU activity in each step. A polyclonal antibody (pAb) against the purified AFU was obtained by anion exchange chromatography on DEAE-52 after ammonium sulfate fractionation and ultrafiltration. Immuohistochemical staining was used to observe the expression of AFU in malignant and adjacent liver tissues.
RESULTS: Human α-L-fucosidase was purified 74–fold to apparent homogeneity with 15% yield. SDS-PAGE indicated the presence of one subunit of molecular weight of 55 Ku. The specific activity of AFU in pooled fraction by chromatography was 10085 IU/mg. Western blot analysis indicated that the pAb could recognize one protein band of molecular weight of 55 Ku. The expression of AFU was observed in cytoplasm membrane of liver cancer tissue but not in that of adjacent tissue.
CONCLUSION: The purified α-L-fucosidase from primary hepatocarcinoma (PHC) is different in its properties from α-L-fucosidase in human other organs. The polyclonal antibody prepared in this experiment can be applied to the diagnosis of PHC.
- Citation: Li C, Qian J, Lin JS. Purification and characterization of α-L-fucosidase from human primary hepatocarcinoma tissue. World J Gastroenterol 2006; 12(23): 3770-3775
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3770.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3770
α-L-fucosidase (AFU) is a glycosidase primarily found in lysosome and involved in the degradation of a variety of fucose-containing fucoglycoconjugates[1,2]. The necessity and importance of AFU (α-L-fucoside fucohydrolase, EC 3.2.1.51) are demonstrated by deficiency of its enzymatic activity leading to fatal neurovisceral storage disorder designated fuocosidosis characterized by mental and motor retardation [3]. The alterations of AFU catalytic activity in human cells, tissues and body fluids have a diagnostic value for human tumors such as primary hepatocarcinoma (PHC)[4-8], colorectal cancer[9-11], ovarian cancer[12].The deficiency of AFU activity in female sera is probably a hereditary condition related to higher risk of ovarian cancer[2,12]. The persistently elevated AFU level in sera of patients with liver cirrhosis contributes to early detection of PHC[5]. In addition, information of the structure and biochemical characteristics of AFU helps to understand the pathogenesis of fuocosidosis on molecular level and to discover therapeutic approaches for the disease[1,13]. In the present investigation, we described an improved purification procedure for AFU by ion exchange chromatography and immunochemical studies using polyclonal antibody against the homogeneous enzyme from human PHC tissue.
Liver specimens were obtained from patients with PHC who underwent partial hepatectomy at Tongji Hospital. The procedure conformed to the local ethical guidelines. Informed consents were obtained from the patients or their relatives. The study protocol followed the ethical guidelines of the Helsinki Declaration of 1975. Rabbits used for antiserum production procedure received human care. All procedures were performed at 4°C unless otherwise specified. Substrate 4-methylumbelliferyl-α-L-fucopyranoside (4-MU-Fuc) and standard product 4-methylumbelliferone (4-MU) were purchased from Sigma. CM-52 and DEAE-52 cellulose resin were purchased from Whatman, USA.
AFU activity was assayed using 4-MU-Fuc as previously described with a minor modification[14-15]. Thirty μL aliquots of enzyme preparation was incubated at 37°C for 30 min with 150 μL of 0.2 mol/L citrate buffer (pH 5.0) containing 60 nmol/L 4-MU-Fuc. The reaction was terminated by addition of 4 mL of 0.2 mol/L K2CO3(pH 10.0). The fluorescent absorbance was read on a HITACHI F-4500 spectrophotometer with excitation at 365 nm and emission peak at 460 nm. Readings were corrected by subtracting tissue and substrate blanks. One unit of enzyme activity was defined as the amount generating 1 nmol of product 4-MU per min at 37°C. Protein concentration was determined by the traditional method of Bradford[16] using Coomassie brilliant blue G-250, following the standard assay and microassay with a protein concentration assay kit (Nanjing, China) using bovine serum albumin as standard protein. To quantify AFU activity in each purification step, we used a modified procedure to determine enzyme activity which assays the activity of β-galactose and generates a standard curve of known product concentration[17].
Crude extract of PHC tissue was prepared as previously described[18] with certain modifications. Portions of human liver from patients with PHC diagnosed by pathological examination were washed in distilled water and cut into small sections, stored in 30% glycerol at -70°C until use. About 25 g of liver tissue (wet weight) was cut into small blocks within 0.05 mol/L cold sodium acetate pH 5.0 (10 mL per gram of tissue, wet weight). The blocks were then homogenized in a glass homogenizer, heated to 37°C for 1 h to release the enzyme. The pH was then immediately adjusted to 5.0 with glacial acetic acid. The mixture was heated to 60°C for 10 min before it was cooled to room temperature on ice and then centrifuged at 0°C for 30 min at 35 000 r/min. The precipitate was discarded and supernatant pH was adjusted to 5.0 with 15 N NH4OH. Solid ammonium sulfate was added to the supernatant to 35% saturation. The solution was allowed to stand at 4°C for 4 h, and then centrifuged at 0°C for 30 min at 29000 r/min. The precipitate was discarded, and the supernatant was saturated to 50% ammonium sulfate by addition of solid ammonium sulfate and allowed to stand at 4°C for 20 h, then centrifuged at 0°C for 30 min at 29000 r/min. The precipitate was dissolved in 4.0 mL of 0.1mol/L sodium citrate (pH 5.0), 0.02% in NaN3 to yield 35%-50% ammonium sulfate fractions. The supernatant was saturated to 60% of ammonium sulfate by addition of solid ammonium sulfate and allowed to stand at 4°C for 20 h, then centrifuged at 0°C for 30 min at 29 000 r/min. The precipitate was dissolved in 2.0 mL of 0.1mol/L sodium citrate (pH 5.0), 0.02% in NaN3 to yield 50%-60 % ammonium sulfate fractions. The 35%-50% and 50%-60% ammonium sulfate fractions were stored at -20°C for chromatography.
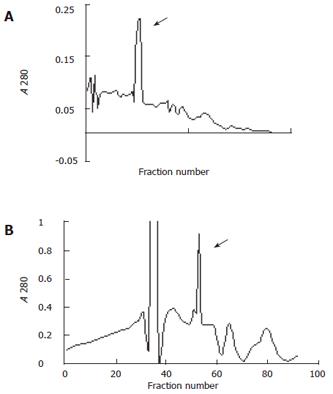
Further fractionations of the 35%-50% and 50%-60% ammonium sulfate fractions were obtained by chromatography on CM-cellulose (Whatman CM-52). To keep the stability of enzyme activity as possible the whole procedure was performed at 4°C. The chromatography column (2.5 cm × 35 cm) was prepared in 0.011 mol/L citric acid-NaOH (pH 4.5), 0.02 % in NaN3 as previously described[18]. Details are given in Figure 1.
The presence of subunits was determined in 12 % polyacrylamide gels (8 cm × 7.3 cm, 0.75 mm) containing 0.1% SDS as previously described[19]. Briefly, 5% stacking and 12 % running gels were run at room temperature using 25 mmol/L Tris-HCl, 0.2 mol/L glycine buffer (pH 8.3) containing 0.1% SDS for 30-40 min at 80 V and 1-1.5 h at 100 V respectively. AFU samples (20-25 μg/lane) were subjected to electrophoresis after treated with 2% SDS, 5% 2-mercaptoethanol, 25% glycerol, 0.1% bromophenol blue in 60 mmol/L Tris-HCl (pH 6.8) in boiling water bath for 3 min. Prestained molecular mass standards (Fermentas, SM0671) were used and stained at the same condition. The gels were stained as previously described[20] with 0.25% Coomassie brilliant blue R-250 in glacial acetic acid: methanol: distilled water (10:45:45, v/v/v) for 3 h, then distained with the mixture of glacial acetic acid : methanol: distilled water (10:45:45, v/v/v) without Coomassie brilliant blue R-250 for 1 h, 20 min per time.
All procedures were performed as previously described[21] unless otherwise specified. Antiserum against AFU was produced by immunizing rabbits with 0.5 mg of purified AFU in equal volume of Freund’s complete adjuvant, and 5 mL serum was collected before immunization as a normal control. The emulsion was injected subcutaneously at multiple sites on the rabbit metapodium palm. After 2 wk, the rabbits were injected with 0.5 mg purified AFU in Freund's incomplete adjuvant intramuscularly in each caudal thigh muscle. After 3 wk, the rabbits were given 0.2, 0.2, 0.4 mg of purified AFU respectively in a week at different sites on ear central artery for booster injection. A blood sample from ear central artery was collected for determination of the tilter after the second immunization. One week after the last booster injection, the rabbits were exsanguinated via cardiac puncture under general anaesthesia using diethyl ether.
Blood was stood at room temperature for 1 h at 4°C overnight, then centrifuged at 13 000 r/min for 30 min at 4°C.The resulting pellet was discarded with the supernatant collected. The whole serum was precipitated with saturated ammonium sulfate to a final saturation of 33%, then desalted with Amicon Ultra-15 PLGC centrifugal filter unit (Millipore, NMWL, 10 KDa) (http://www.millipore.com/catalogue.nsf/docs/C7715).
The desalted antiserum was added to anion exchange column (DEAE-52, Whatman) pre-balanced with 0.005mol/L balancing buffer, pH 8.6, Tris-PO4 and stood at 4°C for 30 min. Fractionations were eluted using 0.055 mol/L (pH 6.0) and 0.5 mol/L (pH5.1) Tris-PO4 by a stepwise developing method, and pooled according to their protein content determined by absorbance of optical density at 280 nm. Because of instability of the purified antibody, chromatography should be carried out at 4°C. Pooled fractionations from DEAE-52 were adjusted to pH 6.4 with 10mol/L NaOH and stored at 4°C to preserve their activity.
The proteins from slab gels were electrotransferred to 0.2 μm-pore-size nitrocellulose membrane (Schleicher and Schuell, Keene, NH) in 48 mmol/L Tirs/HCl transferring buffer containing 39 mmol/L glycine, 0.037% SDS, 20% methanol, at 4°C and 350 mA for 70 min. A portion of nitrocellulose was stained as previously described[22] for 5 min with a working solution of 10-fold dilution of 2% ponceau S, 30% trichloracetic acid (TCA), 30% salicylsulfonic acid, solved in distilled water to 100 mL total volume, then destained vigorously in TBST with shaking until the ponceau S was washed off. The remainder of nitrocellulose was blocked for 2 h under constant shaking at room temperature in 5% non-fat dry milk dissolved in Tris-buffered saline-Tween-20 (TBST) containing 10 mmol/L Tirs/HCl (pH 7.5), 0.15 mol/L NaCl and 0.05% Tween-20. The membrane was incubated overnight at 4°C in 100-fold dilution of immunoglobulin G (IgG) fraction of anti-AFU polyclonal antibody, and washed three times with TBST under constant shaking for 1 h, 20 min per time. The membrane was incubated with the secondary antibody at room temperature for 2 h under constant shaking, 5000-fold dilution of horse-radish peroxide-conjugated immunoPure goat anti-rabbit IgG antibody [IgG (H+L),blotting grade; Pierce]. After three more 20 min washes with shaking (10 mmol/L-Tirs/HCl buffer, pH 7.4), development was accomplished by enhanced chemiluminescence (ECL) for 1 min following the manufacturer’s instructions (Pierce), and the membrane was exposed to Kodak X-ray film. Exposure time was determined on the basis of signals generated by the reaction between membrane and mixture solution from ECL kit. The results were obtained through Kodak medical X-ray processor 102 (Eastman Kodak, Rochester, USA).
The samples were incubated with the primary antibody against AFU (1:50, purified polyclonal, diluted in PBS) at 4°C overnight. SP-immunohistochemistry (SP-IHC) was performed according to the manufacture’s instructions (Zhong Shan Ltd Co, China) for SP kit. Sections were stained with 3, 3’-diaminobenzidine (DAB) and counterstained with haematoxylin for visualization of nuclei. In negative controls, phosphate-buffered saline (PBS) was chosen as the primary antibody instead of anti-human AFU polyclonal antibody.
An effective procedure was developed for the purification of AFU from human primary hepatocarcinoma tissue. The process included homogenization, high speed centrifugation, ammonium sulfate precipitation, ultrafiltration and cation exchange chromatography. The results are summarized in Table1. This procedure typically resulted in a purification of 74-fold with a very high specific activity of 10 085 (nmol.min/mg) protein in 15% yield.
The stability of purified AFU was evaluated following storage at -20°C, 4°C and 20°C (pH 5.0) in the presence of sample buffer respectively. Samples in the frozen condition retained 100% of enzyme activity for at least two months. While samples in refrigerated condition showed slow but progressive decrease of enzyme activity with about 50%-80% of initial activity after two-month storage. Observations also exhibited that 100% enzyme activity remained in aseptic condition at room temperature within two days.
The elution schemes of two fractionations by chromatography are shown in Figure 1.
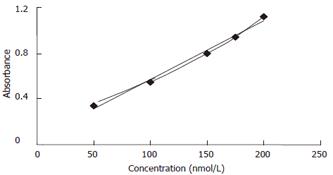
To quantify AFU activity in each purification step, we modified a procedure to assay the activity of β-galactose[17]. Using this method, enzyme activity in unknown samples could be compared according to a standard curve.
The absorbance of known concentrations of commercially available products generated at excitation wavelength 365 nm and emission wavelength 460 nm was shown using HITACHI fluorescence spectrophotometer (Figure 2).
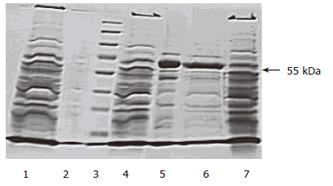
The migration pattern of crude extracts and purified AFU in denaturing polyacrylamide gel electrophoresis is shown in Figure 3. The SDS-PAGE indicated the presence of one subunit of purified AFU at 55 Ku.
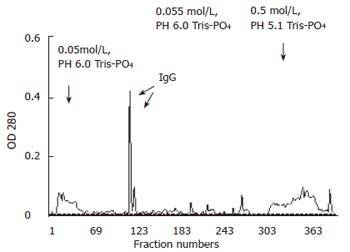
Antiserum was obtained via cardiac puncture by high speed centrifugation, ammonium sulfate precipitation, ultrafiltration and anion exchange chromatography on DEAE-cellulose. The elution profile of anti-serum from anion exchange column is depicted in Figure 4. The curve showed an elution peak of IgG fraction of antiserum against AFU around the 115th collecting tube. SDS-PAGE analysis illustrated the single subunit of 55 kDa of purified IgG and appeared to be highly purified.
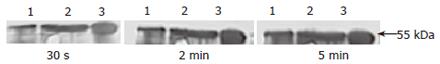
Western blot analysis indicated that purified AFU antiserum recognized the 55 kDa band of the enzyme compared with immunized rabbit serum used as a control. The density of bands seemed to become thicker with prolonged exposure time (Figure 5).
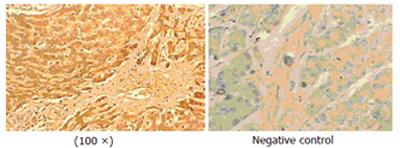
Immunohistochemistry staining indicated that predominant expression of AFU could be seen primarily in plasma membrane and cytoplasm of primary hepatocarcinoma cells compared with adjacent tissue and negative control. This staining was not found in the negative control treated identically except for primary antibody against human liver AFU.
A number of investigations on α-L-fucosidase (EC 3.2.1.51) in different species have provided evidence for its importance in organism metabolism and diseases[1-3]. Although AFU is of considerable interest in human disease research because of fucosidosis, neurovisceral storage disease lacking of its enzymatic activity, many studies on its role in cancer research have been reported. To our knowledge, this is the first report of purification and characterization of human AFU from primary hepatocarcinoma tissue.
In this study, several criteria were chosen to purify and characterize AFU including ammonium sulfate precipitation, ultrafiltration, ion exchange chromatography, SDS-PAGE, Western blot, immunohistochemsitry staining and production of monospecific antibody against purified AFU. The results showed that this procedure was straightforward and successful for AFU purification. Contaminating glycosidases were removed during the course of each purification step and purified enzyme appeared to be homogeneity.
The molecular weight of purified enzyme determined by SDS-PAGE was a single subunit of 55 Ku, which is different from those reported previously[23-27]. These results may prove that purified AFU from PHC tissue is tetrameric, consisting of four identical subunits.
Western blot analysis showed enzyme of the 55 Ku subunit recognized by anti-AFU polyclonal antibody. None of the bands reacted with a preimmune serum (data not shown). This immunochemical recognition is consistent with previous studies on human colon AFU[11], one band of about 55 Ku was detected when incubated with an immunized serum, which is similar to the report of Johnson et al [28].
Immunohistochemistry staining showed predominant expression of AFU in plasma membrane and/or cytoplasm of primary hepatocarcinoma tissue, which is similar to previous results[29] (Figure 6). The precise localization of AFU may suggest that expression level and specific site of AFU expression in primary hepatocarcinoma tissue can be a useful marker for early detection of PHC and a prognosis indicator of PHC development. It was reported that serum AFU activity is a useful marker for early detection of PHC particularly in α-fetoprotein -low or negative PHC patients[4-8,30]. However, the mechanism of elevated AFU in serum or tissues of patients with PHC is still obscure. One possible explanation is the increased synthesis and secretion of AFU by tumor cells[31]. Genetic studies suggest that three different phenotypes of AFU are dominated by two alleles exhibiting autosomal inheritance with a gene dosage effect. Individuals with high activity of AFU in serum are considered homozygous. In addition, sialic acid of glycoproteins determines the clearance rate in circulation and its transformation may cause retarded clearance of glycoproteins[32].
In conclusion, purified AFU and corresponding polyclonal antibody can be used as antigen-antibody candidates to detect PHC at early stage.
The authors thank Hui-Fen Zhu and Xiao-Dan Jiang for their excellent technical assistance, He-Jun Zhou and Wei Yan for preparation of the antiserum.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Johnson SW, Alhadeff JA. Mammalian alpha-L-fucosidases. Comp Biochem Physiol B. 1991;99:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | DiCioccio RA, Barlow JJ, Matta KL. Substrate specificity and other properties of alpha-L-fucosidase from human serum. J Biol Chem. 1982;257:714-718. [PubMed] |
| 3. | Seo HC, Willems PJ, Kretz KA, Martin BM, O'Brien JS. Fucosidosis: four new mutations and a new polymorphism. Hum Mol Genet. 1993;2:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Giardina MG, Matarazzo M, Varriale A, Morante R, Napoli A, Martino R. Serum alpha-L-fucosidase. A useful marker in the diagnosis of hepatocellular carcinoma. Cancer. 1992;70:1044-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Giardina MG, Matarazzo M, Morante R, Lucariello A, Varriale A, Guardasole V, De Marco G. Serum alpha-L-fucosidase activity and early detection of hepatocellular carcinoma: a prospective study of patients with cirrhosis. Cancer. 1998;83:2468-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Deugnier Y, David V, Brissot P, Mabo P, Delamaire D, Messner M, Bourel M, Legall JY. Serum alpha-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma. Hepatology. 1984;4:889-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Bukofzer S, Stass PM, Kew MC, de Beer M, Groeneveld HT. Alpha-L-fucosidase as a serum marker of hepatocellular carcinoma in southern African blacks. Br J Cancer. 1989;59:417-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Leray G, Deugnier Y, Jouanolle AM, Lehry D, Bretagne JF, Campion JP, Brissot P, Le Treut A. Biochemical aspects of alpha-L-fucosidase in hepatocellular carcinoma. Hepatology. 1989;9:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ayude D, Fernández-Rodríguez J, Rodríguez-Berrocal FJ, Martínez-Zorzano VS, de Carlos A, Gil E, Páez de La Cadena M. Value of the serum alpha-L-fucosidase activity in the diagnosis of colorectal cancer. Oncology. 2000;59:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ayude D, Páez De La Cadena M, Martínez-Zorzano VS, Fernández-Briera A, Rodríguez-Berrocal FJ. Preoperative serum alpha-L-fucosidase activity as a prognostic marker in colorectal cancer. Oncology. 2003;64:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Fernández J, Rodríguez-Berrocal FJ, de Carlos A, de Castro G, de la Cadena MP. Nonradioactive immunoquantification of alpha-L-fucosidase protein in human colon tissues. J Biochem Biophys Methods. 1996;31:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Abdel-Aleem H, Ahmed A, Sabra AM, Zakhari M, Soliman M, Hamed H. Serum alpha L-fucosidase enzyme activity in ovarian and other female genital tract tumors. Int J Gynaecol Obstet. 1996;55:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Cragg H, Winchester B, Seo HC, O'Brien J, Swallow D. Molecular basis of the common electrophoretic polymorphism (Fu1/Fu2) in human alpha-L-fucosidase. J Med Genet. 1994;31:659-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Di Matteo G, Orfeo MA, Romeo G. Human alpha-fucosidase. Purification and properties. Biochim Biophys Acta. 1976;429:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Alhadeff JA, Miller AL, Wenger DA, O'Brien JS. Electrophoretic forms of human liver alpha-L-fucosidase and their relationship to fucosidosis (mucopolysaccharidosis F). Clin Chim Acta. 1974;57:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157412] [Article Influence: 3212.5] [Reference Citation Analysis (0)] |
| 17. | A TD-700 Laboratory Fluorometer Method for b-Galactosidase. Turner Biosystems, Inc.. Available from: http://www.turnerdesigns.com/. |
| 18. | Carlsen RB, Pierce JG. Purification and properties of an alpha-L-fucosidase from rat epididymis. J Biol Chem. 1972;247:23-32. [PubMed] |
| 19. | Wang JZ, Fan M. Protein technology Manual. 1st ed. Science Publish Company. 2002;77-110. |
| 20. | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. 2nd ed. Cold Spring Harbor Laboratory Press, 1989. Translated into Chinese by Jin DY, Li MF. Science Publish Company. 1996;885. |
| 21. | Shen GX, Zhou RL. Contemporary immunology experiment technology. 2nd ed. Wuhan: Hubei Science and Technology Publish Company 2002; 23-25, 47-50. |
| 22. | Ed Harlow, David Lane. Using Antibodies: A Laboratory Manual; Translated into Chinese by Shen GX, Gong FL. 2nd ed. Beijing: Science Publish Company 2002; 159-186. |
| 23. | Alhadeff JA, Khunsook S, Choowongkomon K, Baney T, Heredia V, Tweedie A, Bean B. Characterization of human semen alpha-L-fucosidases. Mol Hum Reprod. 1999;5:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Alhadeff JA, Janowsky AJ. Human serum alpha-L-fucosidase. Clin Chim Acta. 1978;82:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Khunsook S, Alhadeff JA, Bean BS. Purification and characterization of human seminal plasma alpha-L-fucosidase. Mol Hum Reprod. 2002;8:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Alhadeff JA, Janowsky AJ. Purification and properties of human brain alpha-L-fucosidase. J Neurochem. 1977;28:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Alhadeff JA, Miller AL, Wenaas H, Vedvick T, O'Brien JS. Human liver alpha-L-fucosidase. Purification, characterization, and immunochemical studies. J Biol Chem. 1975;250:7106-7113. [PubMed] |
| 28. | Johnson SW, Piesecki S, Wang RF, Damjanov I, Alhadeff JA. Analysis of purified human liver alpha-L-fucosidase by western-blotting with lectins and polyclonal and monoclonal antibodies. Biochem J. 1992;282:829-834. [PubMed] |
| 29. | Khunsook S, Bean BS, McGowan SR, Alhadeff JA. Purification and characterization of plasma membrane-associated human sperm alpha-L-fucosidase. Biol Reprod. 2003;68:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wang JJ, Cao EH. Rapid kinetic rate assay of the serum alpha-L-fucosidase in patients with hepatocellular carcinoma by using a novel substrate. Clin Chim Acta. 2004;347:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Takahashi H, Saibara T, Iwamura S, Tomita A, Maeda T, Onishi S, Yamamoto Y, Enzan H. Serum alpha-L-fucosidase activity and tumor size in hepatocellular carcinoma. Hepatology. 1994;19:1414-1417. [PubMed] |
| 32. | Hutchinson WL, Johnson PJ, Du MQ, Williams R. Serum and tissue alpha-L-fucosidase activity in the pre-clinical and clinical stages of hepatocellular carcinoma. Clin Sci (Lond). 1991;81:177-182. [PubMed] |