Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3736
Revised: November 28, 2005
Accepted: December 7, 2005
Published online: June 21, 2006
AIM: To evaluate the histopathological and radiological findings of the gallbladder in patients with autoimmune pancreatitis (AIP).
METHODS: The radiological findings of the gallbladder of 19 AIP patients were retrospectively reviewed. Resected gallbladders of 8 AIP patients were examined histologically and were immunostained with anti-IgG4 antibody. Controls consisted of gallbladders resected for symptomatic gallstones (n = 10) and those removed during pancreatoduodenectomy for pancreatic carcinoma (n = 10), as well as extrahepatic bile ducts and pancreases removed by pancreatoduodenectomy for pancreatic carcinoma (n = 10).
RESULTS: Thickening of the gallbladder wall was detected by ultrasound and/or computed tomography in 10 patients with AIP (3 severe and 7 moderate); in these patients severe stenosis of the extrahepatic bile duct was also noted. Histologically, thickening of the gallbladder was detected in 6 of 8 (75%) patients with AIP; 4 cases had transmural lymphoplasmacytic infiltration with fibrosis, and 2 cases had mucosal-based lymphoplasmacytic infiltration. Considerable transmural thickening of the extrahepatic bile duct wall with dense fibrosis and diffuse lymphoplasmacytic infiltration was detected in 7 patients. Immunohistochemically, severe or moderate infiltration of IgG4-positive plasma cells was detected in the gallbladder, bile duct, and pancreas of all 8 patients, but was not detected in controls.
CONCLUSION: Gallbladder wall thickening with fibrosis and abundant infiltration of IgG4-positive plasma cells is frequently detected in patients with AIP. We propose the use of a new term, sclerosing cholecystitis, for these cases that are induced by the same mechanism as sclerosing pancreatitis or sclerosing cholangitis in AIP.
- Citation: Kamisawa T, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Horiguchi S. Sclerosing cholecystitis associated with autoimmune pancreatitis. World J Gastroenterol 2006; 12(23): 3736-3739
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3736
Autoimmune pancreatitis (AIP) is a unique form of chronic pancreatitis characterized clinically and morphologically by irregular narrowing of the main pancreatic duct, enlargement of the pancreas, increased levels of serum γglobulin, IgG, or IgG4, the presence of autoantibodies, and responsiveness to steroid therapy. Patients with AIP frequently show stenosis of the bile duct system, including the intrahepatic and the extrahepatic bile duct[1-3]. Ultrasonography (US) or endoscopic ultrasonography (EUS) studies have demonstrated the bile duct wall thickening in segments without abnormalities on cholangiography, as well as in stenotic portions[4]. The histopathological findings in the pancreas of AIP are fibrosis and lymphoplasmacytic infiltration with abundant IgG4-positive plasma cells. However, these characteristics are also detected in the bile duct wall of patients with AIP[5,6]. These findings suggest a close relationship between AIP and sclerosing cholangitis[7], though there are only a few studies that have evaluated the gallbladder in AIP[8]. In the present study, we evaluated the histopathological and radiological findings of the gallbladders of AIP patients.
The study subjects were 19 patients with AIP in whom the gallbladder was examined by US and computed tomography (CT) before treatment for AIP. The patients were diagnosed based on the following criteria: (1) irregular narrowing of the main pancreatic duct (n = 19); (2) enlargement of the pancreas (n = 17); (3) pancreatic histology showing dense lymphoplasmacytic infiltration with fibrosis (n = 8); (4) elevation of serum γglobulin (≥ 2.0 g/dL, n = 13), IgG (≥ 1800 mg/dL, n = 13), IgG4 (≥ 136 mg/dL, n = 14); (5) presence of autoantibodies (n = 9); and (6) morphological and serological effectiveness of steroid treatment (n = 11). A clinical diagnosis of AIP was made in all patients who in addition to fulfilling criteria 1, met at least 2 of the remaining criteria.
Six patients had a pancreatoduodenectomy, and 2 had a bile duct resection, cholecystectomy, and a choledochoduo-denostomy with a pancreatic biopsy. These 8 patients had obstructive jaundice due to marked stenosis of the extrahepatic bile duct and were suspected of having carcinoma of the head of the pancreas. Stenosis of the extrahepatic bile duct occurred in 16 patients (1 upper bile duct and 15 lower bile ducts).
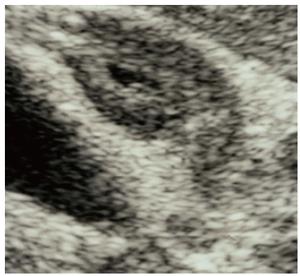
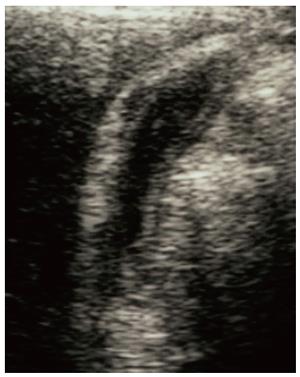
The radiological findings of the gallbladders of all 19 patients were retrospectively reviewed. The degree of thickening of the gallbladder wall was classified into 3 categories: severe (thickness ≥ 8 mm, Figure 1); moderate (thickness from ≥ 4 mm to < 8 mm, Figure 2); and mild or none (thickness < 4 mm).
At least 3 sections of the resected gallbladder of the 8 AIP patients were histologically examined and immunostained by anti-IgG4 antibody (The Binding Site, Birmingham, UK) with avidin-biotin-peroxidase complex (ABC). The pattern of gallbladder inflammation was classified into 2 types: transmural in which the inflammatory infiltrates extended through the gallbladder wall and included involvement and focal destruction of the muscularis (Figure 3); and mucosal-based, in which the inflammatory infiltrates were prominently detected within the lamina propria. The degree of histological thickening of the bile duct wall was classified into 3 categories: severe (thickness ≥ 4 mm); mild (thickness from ≥ 2 mm to < 4 mm); and none (thickness < 2 mm).
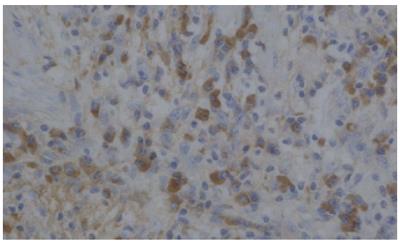
The number of immunohistochemically identified cells per high power field (HPF) in each specimen was counted. The degree of infiltrated IgG4-positive plasma cells was classified as severe (>20/HPF, Figure 4); moderate (10-20/HPF); mild (5-9/HPF); and few (0-4/HPF). The gallbladders resected for symptomatic gallstones (n = 10), those removed during pancreatoduodenectomy for pancreatic carcinoma (n = 10), and the extrahepatic bile ducts and pancreases removed during pancreatoduodenectomy for pancreatic carcinoma (n = 10) were also examined histologically and immunohistochemically as controls.
Thickening of the gallbladder wall was detected on US and/or CT in 10 patients with AIP [3 severe (Figure 1) and 7 moderate (Figure 2)]. All of the 10 patients also had stenosis of the extrahepatic bile duct. The presence of gallstones was noted in only 2 patients.
Histologically, thickening of the gallbladder was detected in 6 of 8 (75%) patients with AIP who underwent surgery; 4 of these 6 patients had transmural lymphoplasmacytic infiltration with fibrosis (Figure 3), and 2 had mucosal-based lymphoplasmacytic infiltration. Lymphoid nodule formation was detected in 2 patients. No patients had dysplastic or neoplastic changes of the gallbladder epithelium. Considerable transmural thickening of the extrahepatic bile duct wall with dense fibrosis and diffuse lymphoplasmacytic infiltration was detected in 7 patients. However, transmural thickening of the gallbladder wall with lymphoplasmacytic infiltration and fibrosis was not detected in the controls.
Immunohistochemically, severe infiltration of IgG4-positive plasma cells was detected in the pancreas of all patients. Severe infiltration of IgG4-positive plasma cells was also detected in the gallbladder (Figure 4) and bile duct wall of 6 patients with thickening of the gallbladder wall. Moderate infiltration of IgG4-positive plasma cells was also detected in the non-thickened gallbladders. In all control cases, IgG4-positive plasma cell infiltration in the gallbladder, bile duct, and pancreas was classified as few (Table 1).
| Thickening of thegallbladder wall | Infiltration of IgG4-positive plasma cells | |||||
| n | Gallbladder wall | Bile duct wall | Pancreas | |||
| Severe | Moderate | Severe | Moderate | Severe | Moderate | |
| Transmural: severe | ||||||
| 4 | 4 | 0 | 4 | 0 | 4 | 0 |
| Mucosal-based | ||||||
| 2 | 2 | 0 | 2 | 0 | 2 | 0 |
| None | ||||||
| 2 | 0 | 2 | 1 | 1 | 2 | 0 |
In AIP, stenosis of the bile duct system is detected from 94%[9] to 100%[10] of patients. In most patients, the lower bile duct is narrowed, and the intrahepatic bile duct or upper bile duct is occasionally involved. Histologically, dense lymphoplasmacytic infiltration with fibrosis, which is the characteristic finding in the pancreas of AIP patients, is also detected in the transmural bile duct wall of many patients with AIP[5,6]. Immunohistochemically, abundant infiltration of IgG4-positive plasma cells is specifically detected in the pancreas of AIP, but the infiltration is also detected in the bile duct wall of AIP patients[5,6]. Bile duct involvement in AIP is considered to be sclerosing cholangitis, which is induced by the same mechanism as sclerosing pancreatitis[7].
According to a report[8] on the pathology of gallbladder lesions in patients with AIP, 12 of 20 (60%) cases of AIP showed intense inflammatory infiltrates, and 7 cases (35%) showed transmural chronic cholecystitis. In the present study, severe or moderate thickening of the gallbladder wall was radiologically detected in 10 of 19 (53%) AIP patients; in these 10 patients, stenosis of the extrahepatic bile duct was also observed. Histologically, thickening of the gallbladder wall was detected in 6 of 8 (75%) AIP patients who underwent surgery; 4 of the 6 showed transmural inflammation and 2 showed mucosal-based inflammation. These 6 patients also showed severe transmural thickening of the extrahepatic bile duct wall. Immunohistochemically, severe or moderate infiltration of IgG4-positive plasma cells was detected in the gallbladder, bile duct, and pancreas of all 8 AIP patients in whom surgery was done, but was not detected in controls. These findings suggest that thickening of the gallbladder wall is one of the extrapancreatic lesions that is frequently detected in AIP patients and is characterized by transmural wall thickening with abundant infiltration of IgG4-positive plasma cells. Thus, we propose the use of a new term, sclerosing cholecystitis, to describe this condition that is induced by the same mechanism as sclerosing pancreatitis or sclerosing cholangitis in AIP.
In conclusion, sclerosing cholangitis is one of the extrapancreatic lesions that is frequently detected in AIP patients and is characterized by transmural wall thickening with abundant infiltration of IgG4-positive plasma cells.
S- Editor Wang J L- Editor Zhao JB E- Editor Ma WH
| 1. | Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Morphological changes after steroid therapy in autoimmune pancreatitis. Scand J Gastroenterol. 2004;39:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Hirano K, Shiratori Y, Komatsu Y, Yamamoto N, Sasahira N, Toda N, Isayama H, Tada M, Tsujino T, Nakata R. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 6. | Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, Okamoto T, Nomura T, Joh T, Itoh M. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20-25. [PubMed] |
| 8. | Abraham SC, Cruz-Correa M, Argani P, Furth EE, Hruban RH, Boitnott JK. Lymphoplasmacytic chronic cholecystitis and biliary tract disease in patients with lymphoplasmacytic sclerosing pancreatitis. Am J Surg Pathol. 2003;27:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55:494-499. [PubMed] |