Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3716
Revised: October 28, 2005
Accepted: November 18, 2005
Published online: June 21, 2006
AIM: To assess if a specific cytotoxic T cell response can be induced in patients with malignant liver tumors treated with radio-frequency ablation (RFA).
METHODS: Six Patients with liver metastases of colorectal cancer and 6 with hepatocellular carcinoma (HCC) underwent RFA. Blood was sampled before, 4 and 8 wk after RFA. Test antigens were autologous liver and tumor lysate obtained from each patient by biopsy. Peripheral T cell activation was assessed by an interferon gamma (IFNγ) secretion assay and flow cytometry. T cells were double-stained for CD4/CD8 and IFNγ to detect cytotoxic T cells. The ratio of IFNγ positive and IFNγ negative T cells was determined as the stimulation index (SI). To assess cytolytic activity, T cells were co-incubated with human CaCo colorectal cancer and HepG2 HCC cells and release of cytosolic adenylate kinase was measured by a luciferase assay.
RESULTS: Before RFA SI was 0.021 (± 0.006) for CD4+ and 0.022 (± 0.004) for CD8+ T cells against nonmalignant liver tissue and 0.018 (± 0.005) for CD4+ and 0.021 (± 0.004) for CD8+ cells against autologous tumor tissue. Four weeks after RFA SI against tumor tissue increased to 0.109 (± 0.005) for CD4+ and 0.11 (± 0.012) for CD8+ T cells against HCC, and to 0.115 (± 0.031) for CD4+ and 0.15 (± 0.02) for CD8+ cells for colorectal metastases (P < 0.0001). No increased SI was observed with nonmalignant tumor tissue at all time points. Before RFA cytolytic activity against the respective cancer cells was low with 2.62 (± 0.37) relative luminescence units (RLU), but rose more than 100 fold 4 and 8 wk after RFA. Spontaneous release was < 2% of maximum release in all experiments.
CONCLUSION: Patients with primary and secondary tumors of the liver show a significant tumor-specific cytotoxic T-cell stimulation with a dramatically increased tumor specific cytolytic activity of CD8+ T cells after RFA.
- Citation: Hänsler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, Strobel D. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol 2006; 12(23): 3716-3721
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3716
Open surgery is still the gold standard for the treatment of hepatocellular carcinoma (HCC) and liver metastases of colorectal cancer. However, more than 75% of these patients are non-resectable for various reasons, such as size and location of the tumor. Radio-frequency thermal ablation (RFA) is a common therapy option for unresectable liver tumors. Thus it was shown that RFA and laser-induced thermo-therapy (LiTT) can achieve survival times comparable to surgery in selected patients[1-3]. RFA uses a radio-frequency current to induce coagulation necrosis which results in a local thermal necrosis accompanied by local inflammation around the necrotic center and by a hemorrhagic rim with lymphoplasmacellular infiltrates[4]. This rim is supposed to be a hot spot for recruitment and activation of tumor-specific T lymphocytes. By using the VX2 hepatoma model in rabbits we could show that RFA can induce a strong mononuclear infiltration around the implanted tumor. It remained unclear if the observed T cell response was merely directed to papilloma viral antigens that are derived from the VX2 hepatoma compared to a tumorspecific T-cell reaction. However, we could recently demonstrate a marked tumor-specific peripherial T cell response in RFA-treated vs untreated rabbits with the VX2 hepatoma[5].
The aim of the present study was to demonstrate if an antitumor T cell response is also inducible in patients who are subjected to RFA. Therefore, we studied the cytotoxic (CD8+ and IFNγ+) T cell and T-helper cell (CD4+ and IFNγ+) response of peripheral blood mononuclear cells (PBMC) against autologous nontumoral and tumoral liver tissue in patients with primary and secondary neoplasms of the liver. In addition, we measured the cytolytic activity of the CD8+ and IFNγ+ T cells against CaCo colorectal cancer and HepG2 HCC cells.
The study was approved by the ethical committee of the University of Erlangen-Nuremberg and performed according to the declaration of Helsinki. All treated hepatocellular carcinomas and colorectal cancer metastases were confirmed histologically prior to therapy. All patients enrolled into the study signed an informed consent prior to inclusion.
In this preliminary group patients with up to 3 tumor nodules within the liver, with a maximum diameter of 6 cm per lesion, were enrolled in the study. Prior local ablative therapy (LiTT, RFA, ethanol injection) or prior chemoembolisation of the malignant liver tumor was an exclusion criterion. Additionally, a curative option by resection or liver transplantation was ruled out. Patients with at least one of the following findings were also excluded: Karnofsky index < 60, thrombocytes < 50 000μL, prothrombin activity < 50%, partitial thromboplastin time > 80 s. No transfusion of platelets or fresh frozen plasma was allowed. Informed consent was obtained from every patient no later than 24 h before treatment.
The size and number of tumor nodules were determined sonographically and by means of computerized tomography (dynamic spiral CT with iv application of contrast medium) prior to RFA.
Twelve consecutive patients (10 male, 2 female) with 14 tumors who met the inclusion criteria were enrolled. Mean patient age was 74 years (range 59 to 80 years). Six patients with six tumors had liver metastases from colorectal cancer, six patients with eight tumors had HCC (Table 1). All patients with HCC had underlying liver cirrhosis (Child A: n = 4, Child B: n = 2) due to chronic hepatitis C (n = 2) or alcohol (n = 4). Mean tumor size was 32 mm (range 10 to 51 mm) with two tumors up to 20 mm, six between 21 and 40 mm and six larger than 40 mm. Mean tumor diameter was 35 mm for HCC (range 24 to 45) and 30 mm for the liver metastases (range 10 to 51mm)
| Tumor entity | CP | Etiology ofcirrhosis | SI before | SI 4 wk | SI 8 wk | |||
| RFA | after RFA | after RFA | ||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |||
| HCC 1 | A | alcohol | 0.012 | 0.017 | 0.086 | 0.125 | 0.15 | 0.146 |
| HCC 2 | A | Hep C | 0.021 | 0.019 | 0.16 | 0.138 | 0.176 | 0.136 |
| HCC 3 | B | Hep C | 0.016 | 0.031 | 0.11 | 0.102 | 0.155 | 0.158 |
| HCC 4 | A | alcohol | 0.015 | 0.019 | 0.07 | 0.11 | 0.079 | 0.112 |
| HCC 5 | B | alcohol | 0.026 | 0.021 | 0.069 | 0.118 | 0.099 | 0.139 |
| HCC 6 | A | alcohol | 0.02 | 0.02 | 0.16 | 0.125 | 0.146 | 0.134 |
| Mean (SD) | 0.018 (± 0.005) | 0.021 (± 0.005) | 0.109b (± 0.042) | 0.120b (± 0.012) | 0.134b (± 0.037) | 0.138b (± 0.015) | ||
| Tumor entity | Primary | SI before | SI 4 wk | SI 8 wk | ||||
| RFA | after RFA | after RFA | ||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |||
| CRC metastasis 1 | Sigma | 0.025 | 0.023 | 0.123 | 0.158 | 0.129 | 0.155 | |
| CRC metastasis 2 | Colon | 0.035 | 0.029 | 0.155 | 0.176 | 0.199 | 0.143 | |
| CRC metastasis 3 | Colon | 0.01 | 0.017 | 0.069 | 0.11 | 0.126 | 0.125 | |
| CRC metastasis 4 | Sigma | 0.016 | 0.018 | 0.099 | 0.127 | 0.112 | 0.126 | |
| CRC metastasis 5 | Rectum | 0.011 | 0.02 | 0.103 | 0.183 | 0.175 | 0.176 | |
| CRC metastasis 6 | Colon | 0.02 | 0.021 | 0.14 | 0.147 | 0.149 | 0.137 | |
| Mean (SD) | 0.019 (± 0.009) | 0.021 (± 0.004) | 0.115b (± 0.031) | 0.150b (± 0.028) | 0.148b (± 0.033) | 0.144b (± 0.019) | ||
The whole procedure was performed under ultrasound guidance (Elegra Advanced®, Siemens, Erlangen, Germany) under sterile conditions. The proposed puncture site was infiltrated with a local anesthetic (2% mepivacain hydrochloride) and the perfused radio-frequency (HF) needle (Berchtold, Tuttlingen, Germany) advanced into the tumor under sonographic guidance. Midazolam (0.5-5 mg) and/or pethidin (25-100 mg) were administered iv as necessary. Patients were monitored by pulse oximetry during the entire procedure.
RFA needle applicators of 2 mm (14 G) diameter and a 15 mm active electrode with microbores were used. During HF application (40 W power output) the RFA needle was continuously perfused with isotonic saline via the bore holes. RF energy was delivered by a computer-assisted radiofrequency generator (Elektrotom 106 HF®, Berchtold, Tuttlingen, Germany) and continuous perfusion of the RFA needle was secured by a syringe pump (Pilot C, Fresenius Medical Care, Alzenau, Germany) linked to the RF generator. Perfusion was adjusted as to impedance by means of an electronic interface between generator and perfusor, automatically increasing in response to a rise in impedance (> 400 Ohm). The RF energy was applied for 10-15 min at each needle position, leading to a coagulation zone of 30-35 mm in diameter. Tumors larger than 20 mm were targeted from different applicator positions to create overlapping coagulation zones, in order to treat the entire lesion with a safety margin > 5 mm. Larger tumors were treated with up to 3 simultaneous needle insertions arranged in a triangle (2 to 4 cm) or square (for larger tumors). After completion of the procedure patients were required to rest in bed for 4 h.
Heparinized blood (LI-Heparin 10 mL) was obtained before, 4 and 8 wk after RFA. The samples were stored at 4°C and tests performed < 12 h after sampling. A liver biopsy of nontumorous and tumorous tissue was obtained from every patient directly before RFA and stored at -20°C.
Tissue-lysates were freshly prepared in cold phosphate buffer (50 mmol/L, pH 7.2) using a glass homogenizer as described[5]. The suspension was filtered with a filter tip (pore size 1.2 μm) to adjust the fragment size to less than 1.2 μm. The protein concentration was measured photometrically according to Bradford and adjusted to 1 mg/mL, followed by sterilization at 600 Gy.
Autologous test antigens (nontumorous liver and tumor lysate, 12.5 μg) were added to 250 μL heparinized blood and cultured in a 15 mL conical polypropylene tube for 16 h at 37°C under 50 mL/L CO2. A negative control without addition of antigen lysate was included, while staphylococcal enterotoxin B served as positive control antigen. Thereafter the samples were put on ice and washed with ice cold washing solution (phosphate buffer saline containing 0.5% bovine serum albumin and 2 mmol/L EDTA, pH 7.4) and the cell suspension centrifuged at 300 g for 10 min at 4°C. The cell pellet was resuspended with 80 μL cold culture medium (RPMI 1640 containing 10% human AB serum). “Catch” reagent (20 μL) containing a bivalent CD45 capture and IFNγ binding antibody (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were added, the suspension was kept for 5 min on ice and after adding 5 mL culture medium the cells were incubated in closed roller tubes for 45 min at 37°C. Thereafter 20 μL phycoerythrin (PE) labelled IFNγ detection antibodies diluted 1:5 as well as 10 μL fluoresceine-thiocyanate (FITC) labelled anti-CD 4 or anti-CD8 antibodies diluted 1:400 (both from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were added to the cooled and washed cells and incubated for 10 min on ice. Five mL erythrocyte lysis buffer, containing 0.155 mol/L NH4Cl, 10 mmol/L KHCO3 and 0.1 mmol/L EDTA diluted 1:10, were added for 10 min and the cells centrifuged at 300 g for 10 min. The washed cells were resuspended in 500 μL cold washing buffer and immediately analysed in a flow cytometer (FACSCalibur, BD Biosciences, Heidelberg, Germany) after addition of 0.25 μg propidium iodide in 5 μL destilled water. FACS data were evaluated using WinMDI Version 2.8 (free version by Joseph Trotter).
PBMC containing T cells were isolated in Leukosep separation tubes by density-gradient centrifugation (PAA Laboratories GmbH, Vienna, Austria). After repeated washing with PBS (Biochrom, Berlin, Germany) containing 50 000 IU/liter heparin (Liquemin N 25 000; Roche, Grenzach-Whylen, Germany), cells were adjusted to a concentration of 106 cells/mL and cultured in RPMI 1640 (Biochrom) containing 10% AB serum (heat-inactivated and sterilized at 600 Gy), 5% HEPES buffer, and 1 μg/mL penicillin/streptomycin.
For further experiments 5 Mio PBMC were resuspended in 1.8 mL cold freezing medium, containing 80% AB serum and 20% DMSO (Sigma-Aldrich, Seelze, Germany), and stored at -80°C. After 24 h cells were transfered into liquid nitrogen for long term storage.
For revitalization cells were defrosted in a waterbath at 37°C and were immediately diluted in warm culture medium (RPMI 1640 (Biochrom) containing 10% AB serum (heat-inactivated and sterilized), 5% HEPES buffer, and 1 μg/mL penicillin/streptomycin).
Cytolytic activity of T-cells was measured by an adenylate kinase (AK) release assay. 10 000 target cells were incubated with 1000 effector cells in a final volume of 200 μL fetal calf serum growth medium (FCS-GM) in round-bottom 96-well microtiter plates. After incubation for 4 h at 37°C100 μL of supernatant were harvested and stored at -20°C for further analysis.
The human hepatocellular carcinoma cell line HepG2 and the human colorectal cancer cell line CaCo served as target cells for the patients suffering from HCC and colonic cancer metastases, respectively. All cell-lines were HLA matched (ABO-system) and tested before.
Maximum AK release was obtained by incubating target and effector cells with 1% v/v Triton-X-100, and baseline AK release with medium alone. Baseline release from T cells and tumor cells was < 10% of maximal release in all experiments and subtracted from each value.
The activity of AK was determined by detection of autoluminescence using a luciferase assay (ToxiLight Kit, Cambrex Corporation, NJ, USA). Twenty μL of supernatant were incubated with AK-detection reagent (Cambrex Corporation, NJ, USA) for 5 min at room temperature. The bioluminescence was measured by a luminometer (BD Monolight 3096 Microplate Luminometer, BD Biosciences, Heidelberg, Germany) and expressed as relative luminescence units (RLU).
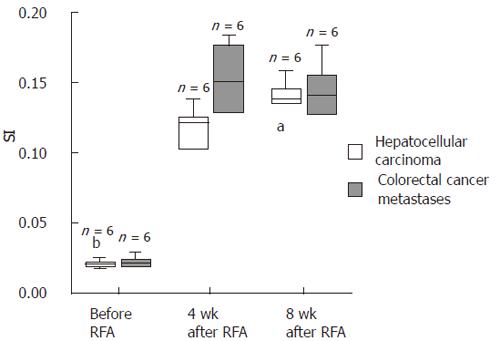
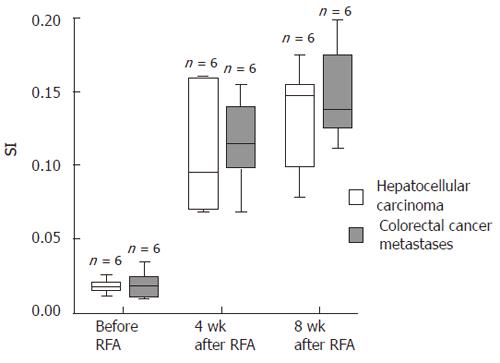
The stimulation index of CD4+ and CD8+ T cells (SI) was determined by the ratio of IFNγ+vs IFNγ- CD4+ and CD8+ T cells, respectively, and calculated with Excel® 2000 software (Microsoft, Seattle, USA). Results were analyzed statistically by the SPSS® software package (Version 11.01 d, SPSS GmbH Software, Munich, Germany). SI values were divided into 2 groups according to the histological origin of the tumors. SI values were calculated numerically (Table 1) and are presented as Box-Whisker plots (Figure 1). The significance of the enhanced SI after RFA treatment was tested with the Wilcoxon test for dependent samples. A P-value less than 0.05 was considered significant.
A total of 26 needle insertions were performed for 12 patients, i.e., 2.2 sessions per patient (range 1 to 3 sessions). Complete remission was achieved in all 12 patients. 2 patients developed a local recurrence within 4 months. One patient could be successfully retreated. The other patient with liver metastases from colorectal carcinoma could not be retreated successfully. Distant recurrences did not occur within the mean follow up-period of 6 months.
The cytokine secretion and capture assay showed that autologous nontumorous control liver tissue did not stimulate IFNγ secretion of circulating T cells before or after RFA. Before RFA: SI[CD4+] = 0.018 (± 0.007) and SI[CD8+] = 0.019 (± 0.004); 4 wk after RFA SI[CD4+] = 0.017 (± 0.005) and SI[CD8+] = 0.019 (± 0.008); 8 wk after RFA: SI[CD4+] = 0.018 (± 0.006) and SI[CD8+] = 0.016 (± 0.007). When exposed to autologous tumor tissue all patients displayed a mean SI[HCC CD4+] of 0.018 (± 0.005) and SI[CRC CD4+] of 0.019 (± 0.009) and SI[HCC CD8+] of 0.021 (± 0.005) and SI[CRC CD8+] of 0.021 (± 0.004) before RFA (Table 1).
Four wk after RFA all patients showed a strong increase of IFNγ+ and CD8+ cytotoxic T cells and CD4+ T helper cells directed against autologous tumor tissue (SI[HCC CD4+] = 0.109 (± 0.042); SI[HCC CD8+] = 0.120 (± 0.012); SI[CRC CD4+] =0.115 (± 0.031); SI[CRC CD8+] = 0.150 (± 0.028)). This increase was highly significant both for the patients with HCC and with colorectal cancer metastases (P < 0.0001) and was maintained until 8 wk after RFA (Table 1, Figures 1 and 2).
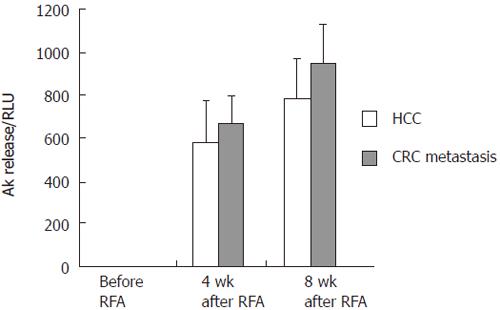
Four wk after RFA PBMC of both groups, HCC and metastasis patients, presented a highly elevated cytotoxic activity with a mean release of adenylate cyclase (AK, as represented by RLU) of 580.24 (± 190.81) and 677.15 (± 138.38) for HCC and colorectal cancer metastasis, respectively. This was maintained 8 wk after RFA with RLU values of 784.96 (± 181.73) and 954.59 (± 275.42). All data were highly significant compared to the baseline before RFA, with 4.42 RLU (± 0.68) for HCC and 2.62 RLU (± 0.37) for colorectal cancer metastasis (P < 0.001). The spontaneous release of AK was always < 2% of the maximum release (Figure 3 and Table 2).
| Tumor entity | CP | Etiology of | AK release before | AK release 4 wk | AK release 8 wk |
| cirrhosis | RFA | after RFA | after RFA | ||
| HCC 1 | A | alcohol | 4.8 | 398.46 | 535.87 |
| HCC 2 | A | Hep C | 3.26 | 421.66 | 645.9 |
| HCC 3 | B | Hep C | 4.88 | 578.63 | 845.99 |
| HCC 4 | A | alcohol | 3.95 | 865.66 | 1025.21 |
| HCC 5 | B | alcohol | 5 | 465.7 | 732.13 |
| HCC 6 | A | alcohol | 4.66 | 751.33 | 924.66 |
| Mean | 4.42 | 580.24b | 784.96b | ||
| (SD) | 0.68 | 190.81 | 181.73 | ||
| Tumor entity | Primary | AK releasebefore | AK release4 wk | AK release8 wk | |
| RFA | after RFA | after RFA | |||
| CRC metastasis 1 | Sigma | 2.13 | 568.32 | 816.55 | |
| CRC metastasis 2 | Colon | 3 | 947.47 | 1298.25 | |
| CRC metastasis 3 | Colon | 2.79 | 627.55 | 945.33 | |
| CRC metastasis 4 | Sigma | 2.56 | 699.45 | 845.54 | |
| CRC metastasis 5 | Rectum | 2.98 | 598.65 | 932.17 | |
| CRC metastasis 6 | Colon | 2.27 | 621.46 | 889.66 | |
| Mean | 2.62 | 677.15b | 954.58b | ||
| (SD) | 0.37 | 139.38 | 175.42 | ||
Thermal treatments such as RFA or LiTT are increasingly used in the therapy of unresectable liver tumors. Survival of treated patients is encouraging. Thus for patients with liver metastases of colorectal cancer, 2 and 5 year survival rates after LiTT of 74% and 30%, respectively,[2,3], and a 2 year survival of 67% after RFA[6] were reported. These data are comparable with results of surgical resection that can lead to 2 and 5 year survival rates of 64% and 28%, respectively, and to disease free survival rates of 35 and 15% for patients with liver metastases of colorectal cancer. Similar results have been obtained for patients with HCC[7,8]. Here again, minimal invasive treatments seem to offer the same survival benefit as open surgery[9,10]. It appears improbable that the achieved results can merely be explained by physical effects like thermal tumor ablation or by a favourable patient selection in case of the nonsurgical group, since patients treated with RFA (or LITT) are often not eligible for hepatic resection due to poor liver function or advanced tumor disease.
Immunotherapy through T cell vaccination or vaccination with dendritic cells is regarded as a promising strategy for the treatment of various tumor entities such as malignant melanomas[11-16], and high expectations have been placed on cytokine-modulated immunotherapies for hepatic tumors[17,18]. It is well known that RFA can induce an unspecific immune stimulation[4]. Thus thermal coagulation causes an inflammatory reaction with lymphoplasmacellular infiltration that can be visualized as a hypervascular rim in contrast CT and contrast-enhanced ultrasound[19]. This hypervascular rim can be so intense as to impede proper assessment of treatment success.
We could recently demonstrate a specific T cell response towards the VX2-tumor implanted in rabbit livers after RFA was performed[5]. The VX2 hepatoma is a papilloma virus-induced tumor[20] which contains viral genomes[21] that are immunogeneic themselves. However, the vigorous T cell reaction was shown not to be directed against viral, but the non-viral tumor antigens. Thus the significantly prolonged survival of RFA-treated animals observed in preliminary studies[5,22,23] supports the hypothesis that tumor immunity induced by in situ vaccination via thermal necrosis may play an important role in the clinical effect of RFA.
It remained to be proven if RFA induces a tumor specific T cell reaction also in humans with hepatic tumors. In the present preliminary study we could clearly demonstrate that patients with HCC or colorectal cancer metastases experienced a 5-6 fold rise of peripheral tumor-specific IFNγ+, CD4+ T helper cells and CD8+ T killer or cytotoxic T cells[24] 4 wk after RFA. Furthermore, this effect was maintained until 8 wk after RFA. The cytotoxic activity of the T cells derived from PBMC showed a dramatical, up to 300 fold increase compared to baseline already 4 and 8 wk after RFA as well.
Two patients with local recurrence (patient HCC4 and CRC 4 in Table 1 and 2) displayed a lower cytotoxic activity against the cancer cells (96.8 and 157.7 RLU 8 months after RFA), as compared to 2 patients without recurrence (patient HCC 2 and CRC 5 with 739.4 and 1004.6 RLU 12 mo after RFA). Due to the low number of cases this observation is not significant and has to be confirmed in a larger group of patients.
Thus it appears that the coagulation of tumor tissue through RFA leads to the enhanced release, exposure and/or denaturation of tumor antigens. Thermally altered tumor antigens are more likely to be phagocytosed by professional antigen presenting cells like dendritic cells[25]. In conjunction with the generation of thermally altered tumor antigens the unspecific inflammatory stimulus induced by RFA might help to overcome immune-tolerance or anergy towards the transplanted tumor, a common phenomenon observed in solid tumors[26,27]. The pro-inflammatory effects of necrotic cells are well documented and appear to be caused by the release of endogenous adjuvants, such as the nuclear protein high mobility group B1 (HMGB1) and heat shock proteins such as hsp70 or gp96[28-30]. RFA thus appears to create an in situ environment resembling T cell vaccination ex vivo.
Our study does not permit conclusions as to whether the stimulation of tumor-specific T cells was solely due to the creation of thermal necrosis, to the instillation of hot saline solution or to the application of a high-frequency current. The relevance and the impact of RFA-induced generation of tumor-specific cytotoxic and NK T cells for potential future multimodal antitumor therapies has to be investigated in prospective randomized multi center trials.
We thank Dr. Heiner Körner (Institute of Molecular Medicine, Nicolaus Fiebiger Center, University of Erlangen-Nuernberg) for making the FACSCalibur and the evaluation software available. We also thank Dorothee Preimel for the gift of Caco cell lines and Katrin Thrumer and Nora Patsenker for the HepG2 cell line.
S- Editor Wang J E- Editor Zhao JB E- Editor Liu Y
| 1. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 602] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 2002;225:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Mack MG, Straub R, Eichler K, Engelmann K, Zangos S, Roggan A, Woitaschek D, Böttger M, Vogl TJ. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom Imaging. 2001;26:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Hänsler J, Neureiter D, Strobel D, Müller W, Mutter D, Bernatik T, Hahn EG, Becker D. Cellular and vascular reactions in the liver to radio-frequency thermo-ablation with wet needle applicators. Study on juvenile domestic pigs. Eur Surg Res. 2002;34:357-363. [PubMed] |
| 5. | Wissniowski TT, Hänsler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496-6500. [PubMed] |
| 6. | Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound. 2001;13:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Nordlinger B, Rougier P. Nonsurgical methods for liver metastases including cryotherapy, radiofrequency ablation, and infusional treatment: what's new in 2001. Curr Opin Oncol. 2002;14:420-423. [PubMed] |
| 8. | Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254-1262. [PubMed] |
| 9. | Livraghi T, Bolondi L, Buscarini L, Cottone M, Mazziotti A, Morabito A, Torzilli G. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian Cooperative HCC Study Group. J Hepatol. 1995;22:522-526. [PubMed] |
| 10. | Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62 Suppl 1:64-68. [PubMed] |
| 11. | Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161-2169. [PubMed] |
| 12. | Couzin J. Cancer immunotherapy. Select T cells, given space, shrink tumors. Science. 2002;297:1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Schuler G, Thurner B, Romani N. Dendritic cells: from ignored cells to major players in T-cell-mediated immunity. Int Arch Allergy Immunol. 1997;112:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Schuler-Thurner B, Dieckmann D, Keikavoussi P, Bender A, Maczek C, Jonuleit H, Röder C, Haendle I, Leisgang W, Dunbar R. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492-3496. [PubMed] |
| 15. | Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 880] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 17. | Kountouras J, Boura P, Kouklakis G. Locoregional immunochemotherapy in hepatocellular carcinoma. Hepatogastroenterology. 2002;49:1109-1112. [PubMed] |
| 18. | Sato T. Locoregional immuno(bio)therapy for liver metastases. Semin Oncol. 2002;29:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Hänsler J, Frieser M, Schaber S, Kutschall C, Bernatik T, Müller W, Becker D, Hahn EG, Strobel D. Radiofrequency ablation of hepatocellular carcinoma with a saline solution perfusion device: a pilot study. J Vasc Interv Radiol. 2003;14:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kidd JG, Rous P. A transplantable rabbit carcinoma originating in a virus-induced papilloma and containing the virus in masked or altered form. J Exp Med. 1940;71:813-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Georges E, Breitburd F, Jibard N, Orth G. Two Shope papillomavirus-associated VX2 carcinoma cell lines with different levels of keratinocyte differentiation and transplantability. J Virol. 1985;55:246-250. [PubMed] |
| 22. | Miao Y, Ni Y, Mulier S, Yu J, De Wever I, Penninckx F, Baert AL, Marchal G. Treatment of VX2 liver tumor in rabbits with "wet" electrode mediated radio-frequency ablation. Eur Radiol. 2000;10:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Hänsler J, Neureiter D, Wasserburger M, Janka R, Bernatik T, Schneider T, Müller W, Frieser M, Schaber S, Becker D. Percutaneous US-guided radiofrequency ablation with perfused needle applicators: improved survival with the VX2 tumor model in rabbits. Radiology. 2004;230:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Pittet MJ, Zippelius A, Speiser DE, Assenmacher M, Guillaume P, Valmori D, Liénard D, Lejeune F, Cerottini JC, Romero P. Ex vivo IFN-gamma secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J Immunol. 2001;166:7634-7640. [PubMed] |
| 25. | Harding CV. Class I MHC presentation of exogenous antigens. J Clin Immunol. 1996;16:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased serum levels of macrophage inflammatory protein-3alpha in hepatocellular carcinoma: relationship with clinical factors and prognostic importance during therapy. Int J Mol Med. 2003;11:601-605. [PubMed] |
| 27. | Hsu TH, Fidler ME, Gill IS. Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology. 2000;56:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Bustin M. At the crossroads of necrosis and apoptosis: signaling to multiple cellular targets by HMGB1. Sci STKE. 2002;2002:pe39. [PubMed] |
| 29. | Heike M, Weinmann A, Bethke K, Galle PR. Stress protein/peptide complexes derived from autologous tumor tissue as tumor vaccines. Biochem Pharmacol. 1999;58:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52:226-234. [PubMed] |