Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3496
Revised: February 10, 2006
Accepted: February 18, 2006
Published online: June 14, 2006
Primary canalicular bile undergoes a process of fluidization and alkalinization along the biliary tract that is influenced by several factors including hormones, innervation/neuropeptides, and biliary constituents. The excretion of bicarbonate at both the canaliculi and the bile ducts is an important contributor to the generation of the so-called bile-salt independent flow. Bicarbonate is secreted from hepatocytes and cholangiocytes through parallel mechanisms which involve chloride efflux through activation of Cl- channels, and further bicarbonate secretion via AE2/SLC4A2-mediated Cl-/HCO3- exchange. Glucagon and secretin are two relevant hormones which seem to act very similarly in their target cells (hepatocytes for the former and cholangiocytes for the latter). These hormones interact with their specific G protein-coupled receptors, causing increases in intracellular levels of cAMP and activation of cAMP-dependent Cl- and HCO3- secretory mechanisms. Both hepatocytes and cholangiocytes appear to have cAMP-responsive intracellular vesicles in which AE2/SLC4A2 colocalizes with cell specific Cl- channels (CFTR in cholangiocytes and not yet determined in hepatocytes) and aquaporins (AQP8 in hepatocytes and AQP1 in cholangiocytes). cAMP-induced coordinated trafficking of these vesicles to either canalicular or cholangiocyte lumenal membranes and further exocytosis results in increased osmotic forces and passive movement of water with net bicarbonate-rich hydrocholeresis.
- Citation: Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol 2006; 12(22): 3496-3511
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3496
Hepatocytes and cholangiocytes are the epithelial cells in the liver, and they both participate in the production of bile. The hepatocytes, the major liver cell population (65%), generate the primary bile at their canaliculi[1]. As an important complement, the epithelial cells lining intrahepatic bile ducts or cholangiocytes (5% of the liver cell population) exert a series of reabsorptive and secretory processes which dilute and alkalinize the bile flow during its passage along the biliary tract[2-6]. Modifications of ductal bile appear to be tightly regulated by the action of nerves, biliary constituents, and some peptide hormones like secretin. Accordingly, it is possible to distinguish between three different bile flow fractions: (1) the canalicular bile salt-dependent flow (30% to 60% of spontaneous basal bile flow) that is driven by concentrative secretion of bile acids by the hepatocytes followed by a facilitated efflux of water[7,8]; (2) the canalicular bile salt-independent flow (another 30% to 60% of spontaneous basal bile flow)[1,8-10], which is also created by hepatocytes but through active secretion of both inorganic and organic compounds (mainly bicarbonate[11,12] and glutathione[13], respectively); and (3) the ductal bile flow, that is the bile salt-independent flow fraction modified and contributed by cholangiocytes, mainly through production of a bicarbonate-rich fluid in response to secretin[2,14-17] and other regulatory factors[2,5,6,17,18].
In the last decade, the use of molecular- and cell-biology tools and the availability of suitable experimental models have greatly facilitated our knowledge on the processes involved in bile generation and modification. This review summarizes some of the experimental models employed in biliary studies, and focuses on the biliary excretion of bicarbonate, the chief factor responsible for ductal bile alkalinization and fluidization, and the role and interactions of regulatory factors.
Biliary studies started over one hundred years ago. In 1902 Bayliss and Starling discovered the hormone secretin as an agent with stimulatory effects on pancreatic secretion and bile flow[19]. About 25 years later secretin was reported as a cholagogue, i.e. an agent capable of stimulating the flow of bile into the duodenum[20], as well as a choleretic agent that could stimulate the production of bile in the liver[21]. In spite of this, although the role of hepatocytes for bile generation was widely recognized quite early, the contribution of cholangiocytes to the production of bile with an adequate composition was accepted more recently. Knowledge of the cholangiocyte contribution was facilitated by the availability of experimental models and the development of both in vivo and in vitro sophisticated procedures.
The initial experimental studies on the bile flow and bile composition were carried out by using in vivo models of conscious or anesthetized mammals with biliary fistulas. In the case of total biliary fistulas, there was a major concern because of the interruption of the enterohepatic circulation and the subsequent depletion of the bile acid pool. However, this can be overcome by continuous intravenous or intraduodenal administration of exogenous bile acids at controlled rates. On the other hand, partial biliary fistula allows controlled interruption of the enterohepatic circulation, and a portion of bile collected can be returned to the stomach or duodenum, thereby replenishing the pool of natural bile acids[10]. Fistula animals have also been useful to estimate canalicular bile flow by measuring the biliary clearance of selected inert solutes (mainly erythritol and mannitol). This procedure assumes that these solutes are sufficiently permeable to enter the canalicular bile by passive processes while being unable to cross the ductal epithelium[22,23]. It appears that this might be the case in some animal species but not in all[24]. Moreover, bile duct cannulation allows direct interventions in the biliary tract through retrograde intrabiliary injection[25-28]; this procedure has been employed to obtain animal models of hepatitis[29] and toxic-induced biliary disease[30], as well as for experimental gene therapy[31,32]. Retrograde intrabiliary injection has also been employed to assess the effects of toxic substances or inhibitors on the bicarbonate-rich choleresis upon stimulation. For instance, ursodeoxycholate-induced bicarbonate-rich choleresis has been shown to be sensitive to intrabiliary phenol[33]. More recently, secretion of bicarbonate in secretin-stimulated rats has been found to be sensitive to intrabiliary administration of particular ion-transport blockers[17].
In humans, in vivo assessment of biliary bicarbonate secretion has employed cumbersome maneuvers like nasobiliary drains in hepatic bile ducts[34]. In a context of surgical interventions, invasive procedures similar to those employed in animals (for instance T-tube insertion into the common bile duct[35,36] or percutaneous transhepatic cholangio-drainage[37]) were also used. Recently, non-invasive assessment of biliary bicarbonate secretion was developed by using positron emission tomography (PET)[38]. This imaging technique allows evaluation of baseline and stimulated organ functions after intravenous injection of short half-live positron emitting isotopes[39,40]. Thus, 2-3 min after bicarbonate labeled with carbon-11 (half live of 20.4 min) was given to healthy volunteers, label uptake was observed in the abdominal region corresponding to the liver parenchyma and hepatic hilum. Interestingly, administration of secretin increased bicarbonate uptake in the parenchymal region, this being followed by accumulation of the label in the perihilar area[38]. Currently, the availability of micro-PET systems may also facilitate non-invasive in vivo studies of biliary bicarbonate secretion in small laboratory animals.
Animal models of bile ductal cell hyperplasia have also been developed, mainly in rodents, to study the pathophysiology of bile ducts[41-43]. These models are closely associated with increased secretin-stimulated ductal secretion. The increased responsiveness seems to occur regardless of the procedure employed to obtain the hyperplasia of cholangiocytes: partial hepatectomy[44], chronic feeding of bile acids[45] or α-naphthylisothiocyanate (ANIT)[46], chronic administration of phenobarbital plus CCl4 to rats[47] or just CCl4 in mice[48], or bile-duct ligation (BDL)[41,49]. In contrast, a decreased secretin-stimulated ductal secretion can be observed in BDL rats with ductopenia following interruption of the cholinergic innervation by total vagotomy[50], or selective damage of large (but not small) cholangiocytes by acute feeding with CCl4[51,52].
The development of genetically modified murine models has contributed to ascertain the role of selective genes, like those for the cystic fibrosis transmembrane conductance regulator Cftr[53,54] and the P-glycoprotein PGY3/MDR2-3/ABCB4 Mdr2/Abcb4[55], among others. In Cftr-/- mice, for instance, induction of colitis has been shown to result in increased bile duct injury[53]. In Mdr2/Abcb4-/- mice, a multistep process of bile-duct damage leading to sclerosing cholangitis has been described[55]. Spontaneous mutant animals can also serve as useful in vivo models. The PCK rat, a model of the autosomal recessive polycystic kidney and hepatic disease (PKHD), has been used for studies on possible trigger factors of biliary cystogenesis. This mutant was spontaneously developed in the rat strain Crj:CD/Sprague-Dawley because of a germ line mutation of the Pkhd1 gene[56,57]. The TR- rat model is widely used for studying canalicular secretion of organic anions. This mutant lacks the functional canalicular isoform of the conjugate export pump MRP2/ABCC2 because of one-nucleotide deletion in the Mrp2/Abcc2 gene[58].
Isolated perfused liver preparations are useful experimental models to evaluate the liver effects of single factors in a manner independent of systemic or humoral effects[59]. There are two modalities of these preparations, as the liver can be isolated and perfused in situ or ex situ, i.e. attached to or removed from the animal body. Livers can be isolated from many animal species (rat[1,59], hamster[60], guinea pig[61], cat[62], rabbit[63], dog[64], sheep[65], calf[66], pig[67], and monkey[68]), the isolated perfused rat liver (IPRL) being the model most widely used[69]. All these models allow repeated collection of both the perfusate and the bile, and permit easy exposure of the liver to different concentrations of test substances. Test substances may be given intravascularly through just the portal vein[1,59-61,70], which provides flow to essentially all hepatocytes[71], or through both the portal vein and the hepatic artery (i.e. isolated bivascularly perfused liver)[72,73]. The latter bivascularly perfused model is particularly adequate to investigate ductal physiology because the predominant blood supply to the bile ducts is via the hepatic artery[74,75]. For studies on ductal secretion, test substances and drugs may also be administered intrabiliary (as retrograde intrabiliary injection[76]). Altogether, these in vitro preparations of isolated perfused liver had provided very valuable data on liver physiology and pathophysiology, the regulation of bile secretion included. However, extrapolation of that data to the in vivo situation should be cautious, as in vivo effects of local factors may be tightly influenced by systemic players such as humoral factors and innervation.
Membrane vesicle preparations derived from rat liver have been useful to distinguish transport across the cell membrane from intracellular events. They may be selectively enriched in basolateral or apical plasma membrane, or in intracellular membranes[77]. The first identification of liver anion exchange activity was carried out in canalicular plasma membrane vesicles[78].
While isolated couplets of hepatocytes was particularly useful as primary secretory units to study canalicular secretion[79], the development in the last decade of the model of isolated bile duct units (IBDU), mainly from rat[16,80,81], but also from mouse[82] is making a great contribution to better study the regulation of ductal bile secretion[83]. Both models permit micropuncture for electrophysiological studies as well as video microscopic optical planimetry to determine bile secretion[16,81,84]. Isolated cholangiocytes[85-89] are also widely employed for transport studies. The preparation of different size subpopulations of cholangiocytes and IBDU isolated from specific portions of the rat intrahepatic biliary tree has made it possible to define the functional heterogeneity of cells lining specific sized intrahepatic ducts[2,90]. Furthermore, knockout and mutant animal models are being used to isolate cholangiocytes and bile duct units to carry out different in vitro studies[91-93]. Moreover, studies have been performed in isolated cholangiocytes from patients with cytic fibrosis (i.e. patients with mutations in the CFTR gene)[94,95]. Finally, a major advance is coming from the development of polarized primary cultures of intrahepatic cholangiocytes from both rat[96,97] and humans[98-101].
As the excretion of bicarbonate to bile involves a change in intracellular pH (pHi) and a counterbalance through ion transporters in the responsible epithelial cells, several procedures and maneuvers have been developed to study pHi regulation. The main strategy is based on the pHi recovery towards its initial values after loading cells with acid or alkali. This type of experiment requires techniques for rapid and efficient monitoring of the pHi. Although the most direct and accurate method seems to be the insertion of double-barreled microelectrodes sensitive to [H+]i into the cell, this has important limitations; tested cells should have large dimensions and a special ability to manipulate the electrodes. Thus a valuable alternative is microfluorimetry, a procedure based on the sensitivity of intracellular fluorescent dyes to pHi. The indicator most widely employed is the membrane-permeable compound 2’, 7’-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein-acetoxymethylester (BCECF-AM)[102-104]. Uncharged BCECF-AM rapidly enters the cell, where it is enzymatically cleaved into its charged, membrane impermeant, fluorescent form BCECF. When bound to H+ ions, BCECF exhibits a shift in its emission fluorescence spectrum, and the ratio between the pH-sensitive emission at 495 nm wavelength, and the pH-insensitive (background) emission at the isosbestic point (440 nm) can be estimated. Once proper adjustments are set up (correcting for cellular autofluorescence, minimizing dye leakage, bleaching, or compartmentation, and preventing photodynamic damage to the cells), this methodology allows for continuous measurements of pHi, with a very rapid response time. The technique can be applied to studies with a fluorimeter, flow cytometer, inverted epifluorescent microscope connected to a photon-counting photometer and a TV camera attachment plus a digital image-processing software[105]. An example of successful use of this methodology is the measurement of the Na+-independent Cl-/HCO3- anion exchange (AE) activity in both cell clusters and single cells. After inducing intracellular alkalinization by administration and further withdrawal of the cell-permeant weak acid propionate in Krebs-Ringer bicarbonate buffer (KRB), it is possible to estimate the anion exchange activity as the rate of spontaneous recovery of pHi[106]. Rates of pHi recovery can be measured as δpHi/δt from the tangent to the experimental plot; transmembrane acid fluxes (or equivalent transmembrane base fluxes, i.e. JOH-) are usually calculated as βtot×δpHi/δt, βtot being the total intracellular buffering power in the presence of CO2/HCO3-, estimated as described[107,108].
The collection of additional important methodologies which enabe a continuous progress in bile secretion pathophysiology is still large. Thus, microcomputed tomography[109], scanning and transmission electron microscopy[89,110], immunoelectron microscopy[111], and dual labeled immunogold[112,113] are only a few among those techniques deserving a brief mention. Moreover, concerning molecular biological tools, the techniques of gene silencing through RNA-interference show great potential for clarifying the function of selective genes in bile duct cells. Currently, RNA-interference may be achieved with small interfering RNAs (siRNA) and through microRNAs (miRNA) and short/small hairpin RNA (shRNA)[114]. siRNAs had been used in normal rat IBDU to examine the role of fibrocystin in ciliary morphology and biliary cystogenesis[57] and that of aquaporin-1 (AQP1) in the transport of water by biliary epithelia[115]. Also siRNA experiments had been carried out in cholangiocarcinoma cells to identify factors involved, for example, in the growth of these cells[116] or their resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)[117]. Usual transfection protocols are not very efficient to internalize the siRNAs in cultured cholangiocytes. Viral vectors that incorporate shRNA expression cassettes have thus been developed. Recently, a recombinant adenovirus with this design has been constructed and employed to efficiently infect normal rat cholangiocytes in culture and test the function of AE2/SLC4A2 anion exchanger in these cells[17].
Bile formation is regarded as an osmotic water flow in response to active solute transport. Bile salts are secreted in the canaliculi through a specific export pump referred to as BSEP/SPGP/ABCB11 (from bile salt export pump, sister of P-glycoprotein, and ATP-binding cassette, subfamily B, member 11, respectively)[118-120], allowing for the generation of the bile salt-dependent flow fraction. Remaining bile-salt independent flow fractions can be driven by supplementary solutes secreted at both the canaliculi (the so-called canalicular bile salt-independent flow), and the bile ducts (named as ductal bile salt-independent flow)[1,2,10,14,121]. Estimates for the magnitude of each bile flow fraction in humans are shown in Table 1.
| Bile flow fraction | Flow rate |
| Canalicular bile acid-dependent flow | 0.15-0.16 mL/min |
| Canalicular bile acid-independent flow | 0.16-0.17 mL/min |
| Ductal secretion of bile flow | 0.11 mL/min |
| Daily total bile flow | 620 mL/d |
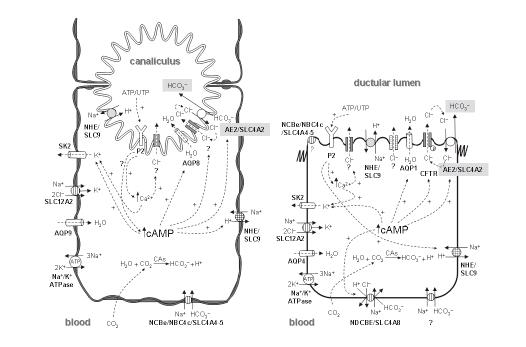
In addition to bile acid secretion, the canalicular membranes of hepatocytes show active secretion of other organic and inorganic compounds, mainly glutathione[13] and bicarbonate[11,12,122], respectively. Glutathione can be secreted via the organic anion transporter MRP2/ABCC2[123], while the efflux of bicarbonate occurs through a DIDS-sensitive Na+-independent Cl-/HCO3- exchange in association with other ion transport systems (Figure 1)[78,124-127]. Both glutathione and bicarbonate seem to have an equivalent major input in canalicular bile flow generation, each driving up to 50% of the bile salt-independent fraction[11]. For this to be accomplished, resultant osmotic forces need to be associated with aquaporin-mediated transcellular movement of water molecules from plasma to the bile canaliculi[128]. In any event, the relative contribution of the canalicular bile salt-independent fraction to the whole canalicular bile flow may vary substantially between animal species (Table 2).
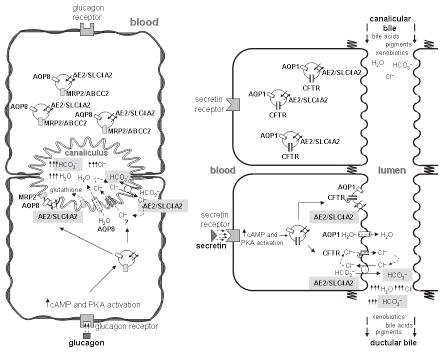
Canalicular bicarbonate excretion has been reported to be regulated by glucagon[134,135], a hormonal oligopeptide secreted by the pancreatic α-cells. This hormone is encoded by the gene GCG, which belongs to the same multigene family as secretin (SCT), vasoactive intestinal peptide (VIP), and gastric inhibitory peptide (GIP) genes, among others. Following its synthesis in the pancreas, glucagon may reach the liver and stimulate the hepatocytes via its interaction with the glucagon receptor (Figure 2). Interestingly, this receptor and the receptors for secretin, VIP, GIP and other small peptidic hormones, are included in a superfamily of receptors characterized by a 7-transmembrane domain structure and by their coupling to adenylate cyclase (ADCY) via GTP-binding proteins (G proteins). Glucagon-glucagon receptor interaction in hepatocytes leads to increased intracellular levels of cAMP, PKA activation, stimulation of canalicular Cl-/HCO3- exchange activity[126,134,135], and enhanced AQP8-mediated water permeability at the canalicular membrane[128,136-138]. Current data strongly suggests that these choleretic effects of glucagon are microtubular-dependent and involve mobilization of intracellular vesicles[126,128,136,139,140]. These effects of glucagon in hepatocytes resemble those of secretin in cholangiocytes (Figure 2). After their interaction with their specific G-protein-coupled receptors, both hormones appear to use a similar cAMP-dependent PKA pathway to co-redistribute cell-type specific intracellular vesicles with flux proteins towards the apical plasma membrane of the target cell. But some of the flux proteins involved in bile formation may differ between hepatocytes and cholangiocytes. While AQP8 is the involved water chanel in hepatocytes[128,136,137,140], it changes to AQP1 in cholangiocytes[112,141,142]. Moreover, there are data suggesting that in hepatic cells in baseline situation, the water channel AQP8, the glutathione carrier MRP2/ABCC2 and the chloride bicarbonate exchanger AE2/SLC4A2[138,140] are present in pericanalicular vesicles that might migrate to the canalicular membrane upon glucagon stimulation[128]. These data, together with previous findings on AE2/SLC4A2 expression at the canaliculi and the pericanalicular area[143,144], point towards the canalicular bicarbonate excretion occurring via an AE2/SLC4A2-mediated Cl-/HCO3- exchange[127]. Although a number of observations suggest that this excretion of bicarbonate in exchange for chloride functions in connection with an apical chloride channel that maintains favorable Cl- gradients[78,124,127], the specific chloride channel(s) involved need yet to be identified (see Figures 1 and 2). While in cholangiocytes AE2/SLC4A2 colocalizes with CFTR[112], this particular cAMP-responsive Cl- channel is not expressed in hepatocytes. Therefore, it might be expected that a Cl- channel other than CFTR[145-148] be physically and/or functionally associated with AE2/SLC4A2 in hepatocytes. Moreover, previous findings have established an important link between bicarbonate excretion to bile and changes in pHi. Canalicular Cl-/HCO3- exchange is a major hepatic acid loading mechanism; at resting pHi and in the absence of any stimulation it shows low activity, but it is rapidly activated by intracellular alkalosis, whereas cAMP stimulation leads to intracellular acidification which requires appropriate counterbalance[124,127]. Figure 1 summarizes several carriers putatively involved in these events in hepatocytes (as well as in cholangiocytes), including acid/base transporters and related proteins. A brief recall on bicarbonate loaders -and therefore acid extruders- highlights the role of electrogenic Na+-HCO3- cotransporters (NBCe)[149-151] and the carbonic anhydrase/CA-CO2 pathway[152,153]. Intracellular load of bicarbonate may increase upon hydration of intracellular CO2 by carbonic anhydrase(s)[154] and subsequent H+ efflux via Na+/H+ exchange (NHE) (Figure 1). The involved exchanger is mainly the basolateral NHE1/SLC9A1[155-157], but NHE4/SLC9A4[158] and the canalicular NHE3/SLC9A3[159] may also participate. The other relevant way of loading bicarbonate into hepatocytes, i.e. through electrogenic Na+-HCO3- cotransport, functions upon membrane depolarization following intracellular acidification[160]. The specific proteins mediating this cotransport in hepatocytes remain to be defined. The only two members of the SLC4 family of bicarbonate transporters[161] known to be electrogenic are NBCe1/SLC4A4 and NBCe2/NBC4/SLC4A5[162]. NBCe1/SLC4A4 is widely expressed in most tissues, but its expression is negligible in the liver[163,164], while mRNA expression for NBCe2/NCB4/SLC4A5 is high in this tissue[165]. Of the known human NBCe2/NCB4/SLC4A5 variants, only the rat NBC4c ortholog can be detected in the rat liver by RT-PCR[164]. Moreover, NBC4c immunoreactivity had been observed at the basolateral membrane of rat hepatocytes[164]. Altogether these findings suggest that the basolateral NBC4c variant might be a relevant bicarbonate loader that enables hepatocytes for canalicular secretion of bicarbonate through the apical AE2/SLC4A2 anion exchanger.
As previously mentioned, primary canalicular bile undergoes a process of fluidization and alkalinization along the biliary tract that is influenced by several factors including hormones (mainly secretin in most species[2,14-17]), innervation/neuropeptides[2,50,166,167], and biliary constitu-ents[2,5,6,17,18]. This process results in net ductal secretion of a bicarbonate-rich watery fluid. The magnitude of the ductal contribution varies between species, representing 30% of basal bile flow in humans and 10% in rats[6]. Cholangiocytes are provided with specific transport systems that participate in bile modifications[2,168]. They are able to take up bile salts via an apical Na+-dependent transporter (SLC10A2, formerly ASBT/ISBT and occasionaly ABAT)[45,169-172] and release them through a basolateral truncated isoform of the same carrier[173]. Indeed, transcellular transport through these carriers is specially important under cholestatic situations. In any case under normal conditions, biliary transport of bicarbonate appears as a relevant function of the bile duct epithelium. It is accomplished by specific acid/base carriers and related transporters that enable cholangiocytes to regulate their pHi[105,174] (Figure 1).
The vectorial transport of HCO3- from cholangiocytes to duct lumen starts with the accumulation of HCO3- in the cells via mechanisms which vary between animal species. In rat cholangiocytes, for instance, HCO3- loading is mediated by transport systems similar to those in hepatocytes, i.e. by electrogenic Na+-HCO3- cotransport activity[168,174-176] and the carbonic anhydrase/CA-CO2 pathway coupled to subsequent carrier-mediated H+ extrusion[153,154]. Carbonic anhydrases (CAs) catalyze the hydratation of carbon dioxide, CO2 + H2O↔ HCO3- + H+ (reviewed in ref. 153). Thus far, several CA isoenzymes have been identified which differ in organ distribution, subcellular location, and function (cf. Table I in ref. 153). Compared with other secretory organs, the mammalian liver contains relatively low levels of total CA activity. Thus, the cytoplasmic CA-II (CA2) is mainly expressed in bile duct cells (but it may also be found in hepatocytes), and appears to be involved in the production of HCO3- for its further secretion to bile[153,177]. Also, the biliary epithelial cells express the membrane associated CA-IV and CA-IX, which on the basis of their location might be involved in acidification and concentration of the bile, even though the exact mechanisms have not been described[153].
Among electrogenic Na+-HCO3- cotransporters[161,162] only the NBC4c variant of NBCe2/NBC4/SLC4A5 was found to be expressed in the rat liver, being immunolocalized to both hepatocytes and cholangiocytes[164]. Interestingly, NBC4c immunoreactivity was basolateral in hepatocytes but apical in cholangiocytes, suggesting a potential role for this cotransporter in the luminal fluid secretion and/or absorption[164]. In humans cholangiocytes, however, Na+-HCO3- cotransport is not active in the physiological range of pHi, being active only at very low pHi, and bicarbonate influx occurs mainly through electroneutral Na+-dependent Cl-/HCO3- anion exchange[176,178]. Thus far, NDCBE/SLC4A8 is the only Na+-dependent Cl-/HCO3- exchanger cloned in humans[179]. Although Northern blot analysis could not detect its messenger RNA in the whole liver[179], this cannot rule out that NDCBE/SLC4A8 be expressed in cholangiocytes (which account for just 5% of the liver cell population).
In both rat and human cholangiocytes, H+ extrusion takes place essentially through NHE activity (Figure 1) -while in pig cholangiocytes H+ extrusion is mediated by a cAMP-activated H+-ATPase[180]. Thus far, several members of the NHE/SLC9 family have been described in rat cholangiocytes. They include NHE1/SLC9A1, restricted to the basolateral membrane and highly sensitive to amiloride, and the amiloride-insensitive isoform NHE2/SLC9A2, which is likely to be active on the side facing the lumen[106]. Moreover, NHE3/SLC9A3 has been immunolocalized to the apical membrane of rat cholangiocytes, where it may play an important role in fluid absorption from the bile duct lumen[159]. Also NHE4/SLC9A4 -an isoform seemingly activated by hypertonicity and with K+/H+ exchange activity[181], was identified in whole liver extracts by Western blot[158]. Some findings in the stomach of Nhe4/Slc9a4-/- mice had led to speculations on a possible functional coupling of NHE4/SLC9A4 with the AE2/SLC4A2 anion exchanger in parietal cells[182]. In any case, the specific cellular expression and function of this NHE isoform in the liver remains to be determined.
As previously reported in hepatocytes, the main acid loader mechanism in bile duct cells is the apical Na+-independent Cl-/HCO3- exchange[17,106,174,176]. Such an AE activity might secrete HCO3- into the lumen which is exchanged for Cl- influx. This exchange is electroneutral, being facilitated by the outside to inside transmembrane gradient of Cl- at relatively high intracellular concentration of HCO3-, specially upon secretin stimulation[16,81,106,175,183] (Figure 2). Actually, several bicarbonate transporters have been described as exerting Na+-independent Cl-/HCO3- exchange activity. This is the case for the SLC4 anion exchangers (AE1/SLC4A1, AE2/SLC4A2 and AE3/SLC4A3)[161,184] as well as several members of the SLC26 gene family of multifunctional anion exchangers (DRA/CLD/SLC26A3, PDS/DFNB4/SLC26A4, and SLC26A6[185] and more controversially SL26A7[186,187]). But none of these carriers except AE2/SLC4A2 had been described to occur in the liver. Moreover, AE2/SLC4A2 was localized not only to the canaliculi but also to the lumenal membrane of bile duct cells[143]. Recent experiments of RNA intereference with recombinant adenovirus expressing shRNA have shown that AE2/SL4A2 is indeed the main effector of both basal and stimulated Na+-independent Cl-/HCO3- exchange in rat cholangiocytes[17].
Besides acid/base transporters cholangiocytes possess other ion carriers like those for Cl-, Na+, and K+, which greatly contribute to pHi regulation and bicarbonate secretion (Figure 1). Thus, the cAMP-responsive Cl- channel CFTR had been localized at the apical side, where it plays a role in biliary excretion of HCO3-[188,189]. Although HCO3- permeability through activated CFTR has been shown in several cell systems[190-194], its main contribution to biliary bicarbonate secretion appears to occur through a coordinated action with AE2/SL4A2[17,175,195]. In addition to CFTR, cholangiocytes possess a dense population of Ca2+-activated Cl- channels. These channels are responsive to interaction of the purinergic-2 (P2) receptors with nucleotides (mainly ATP or UTP)[196-199], and they appear to be related to the Ca2+-calmodulin-dependent protein kinase II[188,200,201]. Resultant Ca2+-stimulated Cl- efflux might be up to 2-fold greater than that mediated by cAMP[202]. Additional high conductance anion channels which are insensitive to both Ca2+ and cAMP were identified in rat bile duct cells[203], but their specific role remains to be defined. The efflux of Cl- from cholangiocytes to the ductular lumen is counterbalanced not only via apical Cl-/HCO3- exchange but also through other Cl- uptake systems, mainly the basolateral Na+-K+-2Cl- cotransporter NKCC1/SLC12A2[196,204]. In addition to maintain high intracellular Cl- concentration facilitating apical Cl- excretion, this forskolin-stimulable cotransporter has been reported to participate in cell volume homeostasis[205] and cell proliferation[204,206,207]. Because the NKCC1/SLC12A2-mediated influx of Cl- occurs together with the entry of Na+ and K+, cholangiocytes possess other systems that sustain the gradients of these cations and the membrane potential difference. Thus a basolateral sodium pump (the Na+/K+-ATPase)[208,209], extrudes Na+ and uptakes K+ with a 3:2 stoichiometry[210]. Accumulation of K+ within cholangiocytes is prevented by its exit through K+ channels. Intracellular cAMP and/or Ca2+ concentration may activate basolateral K+ conductance that hyperpolarizes the cell[211]. The small conductance K+ channel SK2/KCNN2 has been identified in rat and human hepatocytes[212] and in rat and human biliary epithelia[202,212], the activity of which is stimulated by small increases in intracellular Ca2+ in an apamin-sensitive manner[213]. Although SK2/KCNN2 immunoreactivity has been localized in cholangiocytes at both apical and basolateral membranes, functional studies in polarized preparations have demonstrated a significantly greater basolateral Ca2+-stimulated K+ conductance[202]. Because the nonselective K+ channel blocker Ba2+ is able to cause a greater degree of inhibition than apamin[202], apamin-insensitive channels not yet identified may also be involved in the conductance of K+.
In a context of cAMP- and/or Ca2+-stimulated Cl- channels, cell hyperpolarization due to K+ conductance facilitates the transcellular Cl- movement into the lumen. Recycling of K+ can be held up through the basolateral Na+-K+-2Cl- cotransport and the Na+/K+-ATPase. All these ionic fluxes across cholangiocyte membranes may ultimately lead to biliary excretion of bicarbonate via its exchange with lumenal chloride, which is facilitated by the outside to inside transmembrane gradient of chloride at relatively high intracellular concentration of bicarbonate[16,81,106,175,183]. The apical fluxes of anions result in increased osmotic forces in the bile duct lumen which in the presence of aquaporins contribute to the generation of ductal bile flow. This view has been strongly supported by the finding that AE2/SLC4A2 and CFTR both colocalize with AQP1 in cholangiocyte intracellular vesicles which co-redistribute to the apical cholangiocyte membrane upon both cAMP and secretin stimulations[112].
Aquaporins are water channels that mediate a bidirectional passive movement of water molecules across epithelial cells in response to osmotic gradients established by ions and solutes. Thus far, rodent cholangiocytes have been described to possess two types of aquaporins. Thus, in addition to the AQP1 locating in the intracellular vesicles which may traffic to the apical membrane upon agonist stimulation[112], there occurs another water channel at the basolateral membrane of cholangiocytes named AQP4, which is not sensitive to secretin[214,215] (Figures 1 and 2). However, there can be differences between species. For instance, immunohistochemical analysis in liver pig has shown the presence of AQP1 and AQP9 in cholangiocytes, while AQP3, AQP4, AQP7, and AQP8 were absent in these cells[216].
The bile duct epithelium is constantly regulated by the action of multiples factors that contribute to the formation of bile flow with an adequate final composition. Among these factors secretin is a relevant hormone peptide which may induce bicarbonate-rich hydrocholeresis in many animal species. Thus, secretin regulation of ductal bile flow and the concurrence of other factors are briefly summarized as follows:
In 1902, Bayliss and Starling[19] described an active agent originating from the intestinal mucosa, which they referred to as secretin because of its capacity to stimulate pancreatic secretion in the dog. They also used the word “hormone” to designate this sort of compound that can be produced in an organ and carried through the circulation to exert its effect on another organ. Some years passed before the choleretic effect of secretin in the liver was reported in several animal species and humans[217-219]. Secretin was found to stimulate bile flow together with a decrease in the concentration of bile salts[217-219]. In the late 1960s the role of bile ducts in secretin-induced hydrocholeresis was postulated because of the observation that this secretin effect was associated with reciprocal changes in the biliary concentration of bicarbonate and chloride anions[35].
Meanwhile secretin was purified from most species, first in 1962 from pig[220,221] and then from humans, dog, goat, guinea pig, rabbit, rat, mouse and chicken. Secretin has only 27 amino acids, which allowed for its chemical synthesis as early as 1968[222]. There is close homology between mammalian secretins, but also between the regulatory peptides (more than 10) currently grouped in the secretin/glucagon/ VIP superfamily.
Secretin is produced in many organs but mainly in the mucosa of upper small intestine (duodenum and jejunum)[223,224]. Its release to blood occurs mainly in the postprandial period, being stimulated by gastric acid delivered into duodenal lumen[225], as well as by pancreatic and intestinal secretions[226,227]. Secretin release appears to be mediated by a luminal secretin-releasing peptide contained in these gastrointestinal juices[228-230]. In addition to its mentioned bilary and pancreatic effects, secretin may function as feedback inhibitor of gastrin (GAST) release and gastric acid secretion[231], and may also regulate gastric motility[232]. The physiological activities of secretin are subjected to hormone-hormone and neuro-hormonal interactions. Inhibition of gastric acid secretion by secretin is thus mediated by somatostatin (SST) and prostagladins[233,234], and secretin inhibition of gastric motility involves a vagal afferent pathway[235,236].
Secretin exerts its physiological actions via interaction with the N-terminal extracellular tail of its specific glycoprotein receptor SCTR[237-239]. Like the glucagon receptor and other members of the same receptor super-family, SCTR is coupled to adenylate cyclase/ADCY through an oligomeric GTP-binding protein. In the liver SCTR is exclusively expressed at the basolateral membrane of cholangiocytes[240,241]. As previously noted, the action of secretin in cholangiocytes runs parallel with the choleretic effect of glucagon in hepatocytes (Figure 2). Thus, both glucagon and secretin may stimulate the bile salt-independent bile flow, but each at a different level, i.e. canalicular for the former and ductular for the latter(cf. ref. 242).
Secretin-SCTR interaction in cholangiocytes results in increased intracellular levels of cAMP[15,16,243]. Further PKA activation[175,183] can induce microtubule-dependent co-redistribution of the intracellular vesicles with AE2-CFTR-AQP1 flux carriers to the apical membrane[112]. Additionally, the CFTR is phosphorylated and activated[244], thus resulting in Cl- efflux to the ductular lumen. In consequence, Cl- secreted by CFTR can be exchanged with HCO3- through AE2/SLC4A2. This mechanistic model has been consistently confirmed in vitro using rat IBDU[16,175] and cholangiocytes[174]. For in vivo studies, most experiments with rats have used some of the aforementioned models with bile duct proliferation[41,43,46,112,245,246], since normal rats appear to respond very poorly to secretin (likewise rabbit, but in contrast with guinea pig among rodent species)[15,41,46,47,247]. Indeed the expression of SCTR is increased in BDL rat cholangiocytes[245,246] but the receptor is also expressed in normal rat cholangiocytes[240]. In the model of IPRL[248], intra-arterial secretin increased biliary concentration of bicarbonate, but had no effect on the net bile flow. Because the effect of secretin was blocked only by the CFTR inhibitor NPPB and not by the anion exchanger inhibitor DIDS (both administered intra-arterially), it was proposed that Cl-/HCO3- exchange would have no role in the ductal secretion of bicarbonate in the normal rat[248]. However, recent experiments indicate that secretin does increase bile flow and biliary Cl- and HCO3- excretions in the normal rat, but when they maintain the bile acid pool via continuous infusion with taurocholate[17]. Moreover, these effects of secretin were distinctively blocked by the inhibitors given by intrabiliary retrograde injection. While secretin effects were all blocked by intrabiliary NPPB, DIDS only inhibited secretin-induced increases in bile flow and bicarbonate excretion but not the increased chloride excretion[17]. These findings provide evidence for the role of biliary Cl-/HCO3- exchange in secretin-induced bicarbonate-rich choleresis in the normal rat model.
In line with former findings in earlier experiments with dogs[14,249], the aforementioned observation that secretin also has effects on the normal rat when infused continuously with taurocholate[17] confirms the notion that bile acids are relevant for secretin actions. In a previous study in normal rats secretin and taurocholate were tested and no secretin effects were observed[41]. But in that study, taurocholate infusion was interrupted before secretin administration[41]. It is already known that bile acids can enter into cholangiocytes through the carrier ASBT[45,169-172] and exert their effects on these cells (reviewed in refs. 2 and 250). For instance, it has been recently reported, activation of CFTR by ASBT-mediated bile salt absorption, which is seemingly independent from cAMP or cGMP signaling[251].
The bile salt-dependent canalicular flow is related to the osmotic activity of bile acids, but some bile acids such as ursodeoxycholic acid (UDCA), 23-nor-UDCA, and 23-nor-chenodeoxycholate[252-254], have a higher choleretic effect than can be accounted for by their secretion into bile (the so-called hypercholeretic effect). This hypercholeretic effect is associated with a marked stimulation of bicarbonate secretion into bile. A classic hypothesis referred to as “cholehepatic shunt pathway”[253] claimed that the hypercholeretic effect may involve intraductal protonation of unconjugated bile salts which results in the formation of bicarbonate anions derived from hydrated CO2 (i.e. H2CO3 or H+ plus HCO3-). Passive diffussion of uncharged bile acids through cholangiocytes to the periductular vessels and further uptake in hepatocytes could be followed by their canalicular resecretion as unconjugated bile salts (reviewed in ref. 250). After the identification of apical and basolateral bile acid carriers in cholangiocytes (ASBT and tASBT, respectively[45,169-173]), the cholehepatic shunt hypothesis has received a boost, being updated for the conjugated bile salts[250]. ASBT activity acutely increases upon secretin stimulation[172], which may accentuate the cholehepatic bile acid shunting in the postprandial period.
The liver is directly regulated by the neurovegetative innervation. Indeed, the release of the neurotransmitter acetylcholine from the intrahepatic parasympatic terminals induces, via selectively interaction with M3 Ach receptors on cholangiocytes, an increase in both secretin-stimulated cholangiocyte cAMP synthesis and Cl-/HCO3- exchanger activity by Ca2+-calcineurin-mediated PKC-independent modulation of adenylate cyclase/ADCY[255,256]. In fact, infusion of acetylcholine in the IPRL model was found to potentiate the effect of secretin on biliary HCO3- excretion[248]. Furthermore, interruption of parasympatic innervation in BDL rats by vagotomy has been reported to inhibit secretin-stimulated ductal secretion, as well as to decrease cholangiocyte intracellular cAMP levels[50].
The intrahepatic biliary epithelium also receives dopaminergic innervation[257,258], but in contrast to the cholinergic system, the dopaminergic system inhibits secretin-induced choleresis. Although both systems exert their functions through increased intracellular ions (1, 4, 5) P3 and Ca2+, the cholinergic system acts via calmodulin and calcineurin but without recruitment of PKC[256], whereas the D2 dopaminergic system inhibits secretin-stimulated ductal secretion through increased expression and activa-tion of PKC-γ[259].
Moreover, cholangiocyte secretion can also be regulated by the action of the adrenergic system[260,261]. The α2-adrenergic receptor agonist UK-14304 has been reported to inhibit cholangiocarcinoma growth through time course-dependent modulation of Raf-1 and B-Raf activities[260], and the α1-adrenergic agonist phenylephrine can potentiate secretin-stimulated ductal secretion through the amplification of the ADCY system via a Ca2+- and PKC-dependent mechanism[261].
In addition to secretin, there are gastrointestinal hormones and neuropeptides such as bombesin/gastrin releasing peptide (BN/GRP), VIP, endothelin-1 (ET1/EDN1), somatostatin/SST, and gastrin/GAST, which may also modulate the ductular bile salt-independent flow (reviewed in ref. 2). Some of these factors operate through a secretin-independent mechanism, while others influence the release of secretin or interact with the secretin signaling in cholangiocytes, depending very much on the animal species. For instance, the neuropeptide bombesin/BN/GRP can act either by increasing the secretin release in dogs[262,263], or inducing ductal secretion with activated Cl-/HCO3- exchange via secretin-independent mechanisms in isolated rat cholangiocytes[2,264-266]. The effect of VIP on cholangiocytes depends also on the animal species[2,267-271]. While VIP appears to increase secretin-stimulated bile flow and bicarbonate excretion in humans[267,268], studies in rat IBDU show that VIP can stimulate basal fluid and bicarbonate secretion through a cAMP-independent pathway[2,269].
The cyclic tetradecapeptide somatostatin/SST is able to inhibit basal and secretin-stimulated bicarbonate-rich choleresis via its interaction with the SSTR2 receptor subtype[2,272]. In rat, inhibition of secretin-stimulated ductal secretion by SST is associated with decreased expression of the secretin receptor SCTR in cholangiocytes and reduced secretin-stimulated cAMP levels[49,272]. In mice SST has also been shown to stimulate ductal fluid absorption, a process involving intracellular cGMP synthesis and inhibition of secretin-stimulated cAMP synthesis[273]. Moreover, in dogs, SST has been shown to diminish the acid-induced release of secretin from the duodenal mucosa[262]. Similarly SST, the gastrointestinal hormone gastrin/GAST may also modulate cholangiocyte secretion[2]. In BDL rats, GAST has been reported to inhibit secretin-stimulated ductal secretion by reducing both SCTR expression and secretin-induced cAMP levels[274].
Other peptide hormones like insulin and insulin-like growth factor 1 (IGF1) can also modulate the biliary epithelium. Insulin was reported to inhibit secretin-induced secretion in BDL rats through activation of PKC and inhibition of secretin-stimulated cAMP and PKA activity[275]. On the other hand, studies using a liver cell line (though from hepatoma rather than cholangiocytic type), showed that insulin may stimulate membrane turnover with increased exocytosis/endocytosis of vesicles containing ion channels[276]. In rats IGF1 was found to stimulate choleresis[277] as well as cholangiocyte proliferation[278]. IGF1 can be synthesized and released from cholangiocytes under the control of the growth hormone (GH)[278]. Biliary IGF1 may in turn interact with its receptor (IGF-R) located at the apical pole of cholangiocytes[278]. Expression of both IGF1-R and IGF1 is markedly enhanced in cholangiocytes following bile duct ligation[278].
Also steroidal hormones like corticosteroids and estrogens have effects on the biliary epithelium. Corticosteroids are choleretic and increase biliary bicarbonate excretion[277,279]. However, estrogen-induced cholestasis results in diminished biliary bicarbonate excretion[280]. Reduced bicarbonate excretion might be caused by a reflux of biliary bicarbonate via leaky tight junctions as it is not associated with impaired activity of the Cl-/HCO3- exchanger[280].
Both hepatocytes and cholangiocytes are able to release ATP and UTP, which leads to increased biliary concentration of nucleotides and nucleosides (the latter being a result of nucleotide dephosphorylation by membrane-associated nucleotidases)[281-283]. Stimulation of the different subtypes of purinergic receptors (PR) at the lumenal membrane of cholangiocytes by either extracellular nucleosides (P1 family receptors) or extracellular nucleotides (P2 family receptors) may control cholangiocyte secretion and ion channel activities[281,283-286]. Most subtypes of purinergic receptors are G protein-coupled receptors except the P2X subtypes which are ligand-gated channels[281,283]. P2 activation stimulates cholangiocyte biliary efflux of K+, HCO3-, and Cl-, and reabsorption of Na+[281,283,286]. Whereas Cl- efflux seems to be mediated by a calcium-stimulated Cl- channel[284], HCO3- is secreted from cholangiocytes via an AE2/SLC4A2 mediated Cl-/HCO3- exchange[17,105,174,284,287]. Of notice, the P2Y11 subtype receptor has been reported to mediate secretion via cAMP in pancreatic duct epithelial cells[288], which suggests that a similar mechanism may also occur in cholangiocytes (as well as in hepatocytes). Finally, P2 receptors may stimulate the basolateral NHE1/SLC9A1 activity in cholangiocytes[287].
Some cytokines such as IL5 and the combination of the proinflammatory cytokines IL6, IFN-γ, IL1, and TNF-α can inhibit secretin-induced choleresis[289,290]. Moreover, proinflammatory cytokines can impair the barrier function of biliary epithelia[290], and stimulate the biliary epithelium to generate NO, via induction of inducible nitric oxide synthase 2A (NOS2A/INOS). Resultant reactive nitrogen oxide species (RNOS) may cause ductular cholestasis through inhibition of both the soluble adenylate cyclase (SAC) and the cAMP-dependent HCO3- and Cl- secretory mechanisms[195]. Such a pathogenetic sequence may contribute to ductal cholestasis in inflammatory cholangiopathies[195].
In conclussion, biliary secretion of bicarbonate is an important contributor to the generation of the bile-salt independent flow. Bicarbonate is secreted from both hepatocytes and cholangiocytes through parallel mechanisms which involve chloride efflux through activation of Cl- channels, and further bicarbonate excretion via AE2/SLC4A2-mediated Cl-/HCO3- exchange. Glucagon and secretin are two relevant hormones which act very similarly in hepatocytes and cholangiocytes, respectively. These hormones interact with their specific receptor, resulting in increased intracellular cAMP levels and activation of cAMP-dependent Cl- and HCO3- secretory mechanisms. Both hepatocytes and cholangiocytes seem to have cAMP-responsive intracellular vesicles in which AE2/SLC4A2 may colocalize with cell specific Cl- channels (CFTR in cholangiocytes and thus far undetermined in hepatocytes) and aquaporins (AQP1 in cholangiocytes and AQP8 in hepatocytes). cAMP-induced coordinated trafficking of these vecicles to either canalicular or cholangiocyte lumenal membranes and further exocytosis results in increased osmotic forces and passive movement of water with net bicarbonate-rich hydrocholeresis.
S- Editor Pan BR E- Editor Bi L
| 1. | Boyer JL. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971;221:1156-1163. [PubMed] |
| 2. | Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612-G625. [PubMed] |
| 3. | Boyer JL. Bile duct epithelium: frontiers in transport physiology. Am J Physiol. 1996;270:G1-G5. [PubMed] |
| 4. | Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol. 1997;272:G1064-G1074. [PubMed] |
| 5. | Baiocchi L, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bile secretion. J Hepatol. 1999;31:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Tavoloni N. The intrahepatic biliary epithelium: an area of growing interest in hepatology. Semin Liver Dis. 1987;7:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Sperber I. Biliary secretion of organics anions and its influence on bile flow. The Biliary System, Oxford: Blackwell 1965; 457-467. |
| 8. | Erlinger S, Dhumeaux D. Mechanisms and control of secretion of bile water and electrolytes. Gastroenterology. 1974;66:281-304. [PubMed] |
| 9. | Wheeler HO, Ross ED, Bradley SE. Canalicular bile production in dogs. Am J Physiol. 1968;214:866-874. [PubMed] |
| 10. | Erlinger S. Bile flow. The liver: biology and pathobiology, New York: Raven Press 1994; 769-786. |
| 11. | Hardison WG, Wood CA. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978;235:E158-E164. [PubMed] |
| 12. | Van Dyke RW, Stephens JE, Scharschmidt BF. Effects of ion substitution on bile acid-dependent and -independent bile formation by rat liver. J Clin Invest. 1982;70:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am J Physiol. 1989;256:G22-G30. [PubMed] |
| 14. | Wheeler HO, Mancusi-Ungaro PL. Role of bile ducts during secretin choleresis in dogs. Am J Physiol. 1966;210:1153-1159. [PubMed] |
| 15. | Lenzen R, Alpini G, Tavoloni N. Secretin stimulates bile ductular secretory activity through the cAMP system. Am J Physiol. 1992;263:G527-G532. [PubMed] |
| 16. | Roberts SK, Kuntz SM, Gores GJ, LaRusso NF. Regulation of bicarbonate-dependent ductular bile secretion assessed by lumenal micropuncture of isolated rodent intrahepatic bile ducts. Proc Natl Acad Sci U S A. 1993;90:9080-9084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Banales JM, Arenas F, Rodríguez-Ortigosa CM, Sáez E, Uriarte I, Doctor RB, Prieto J, Medina JF. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Strazzabosco M, Zsembery A, Fabris L. Electrolyte transport in bile ductular epithelial cells. J Hepatol. 1996;24 Suppl 1:78-87. [PubMed] |
| 19. | Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325-353. [PubMed] |
| 21. | Lueth HC, Kloster G. The effect of purified secretin on bile flow from the liver. Ame J Physiol. 1928;85:389. |
| 22. | Forker EL. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967;46:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Forker EL. Bile formation in guinea pigs: analysis with inert solutes of graded molecular radius. Am J Physiol. 1968;215:56-62. [PubMed] |
| 24. | Tavoloni N. Biliary clearance of inert carbohydrates. Expectations and reality. Gastroenterology. 1988;94:217-228. [PubMed] |
| 25. | Peterson RE, Fujimoto JM. Retrograde intrabiliary injection: absorption of water and other compounds from the rat biliary tree. J Pharmacol Exp Ther. 1973;185:150-162. [PubMed] |
| 26. | Bockman DE. Route of flow and micropathology resulting from retrograde intrabiliary injection of India ink and ferritin in experimental animals. A combined light- and electron-microscopic study. Gastroenterology. 1974;67:324-332. [PubMed] |
| 27. | Olson JR, Fujimoto JM. Evaluation of hepatobiliary function in the rat by the segmented retrograde intrabiliary injection technique. Biochem Pharmacol. 1980;29:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Ballatori N, Jacob R, Boyer JL. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986;261:7860-7865. [PubMed] |
| 29. | Takehara T, Hayashi N, Miyamoto Y, Yamamoto M, Mita E, Fusamoto H, Kamada T. Expression of the hepatitis C virus genome in rat liver after cationic liposome-mediated in vivo gene transfer. Hepatology. 1995;21:746-751. [PubMed] |
| 30. | Goetz M, Lehr HA, Neurath MF, Galle PR, Orth T. Long-term evaluation of a rat model of chronic cholangitis resembling human primary sclerosing cholangitis. Scand J Immunol. 2003;58:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Tominaga K, Kuriyama S, Yoshiji H, Deguchi A, Kita Y, Funakoshi F, Masaki T, Kurokohchi K, Uchida N, Tsujimoto T. Repeated adenoviral administration into the biliary tract can induce repeated expression of the original gene construct in rat livers without immunosuppressive strategies. Gut. 2004;53:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kuriyama S, Yoshiji H, Deguchi A, Nakai S, Ogawa M, Nonomura T, Kimura Y, Inoue H, Kinekawa F, Tsujimoto T. Safe and efficient transgene expression in rat hepatocytes induced by adenoviral administration into the biliary tract. Oncol Rep. 2005;13:825-830. [PubMed] |
| 33. | Dumont M, D'Hont C, Moreau A, Mbape H, Feldmann G, Erlinger S. Retrograde injections of formaldehyde into the biliary tree induce alterations of biliary epithelial function in rats. Hepatology. 1996;24:1217-1223. [PubMed] [DOI] [Full Text] |
| 34. | Knyrim K, Vakil N. The effects of synthetic human secretin on calcium carbonate solubility in human bile. Gastroenterology. 1990;99:1445-1451. [PubMed] |
| 35. | Waitman AM, Dyck WP, Janowitz HD. Effect of secretin and acetazolamide on the volume and electrolyte composition of hepatic bile in man. Gastroenterology. 1969;56:286-294. [PubMed] |
| 36. | Lenzen R, Bähr A, Eichstädt H, Marschall U, Bechstein WO, Neuhaus P. In liver transplantation, T tube bile represents total bile flow: physiological and scintigraphic studies on biliary secretion of organic anions. Liver Transpl Surg. 1999;5:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Fukumoto Y, Okita K, Yasunaga M, Konishi T, Yamasaki T, Ando M, Shirasawa H, Fuji T, Takemoto T. A new therapeutic trial of secretin in the treatment of intrahepatic cholestasis. Gastroenterol Jpn. 1989;24:298-307. [PubMed] |
| 38. | Prieto J, García N, Martí-Climent JM, Peñuelas I, Richter JA, Medina JF. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology. 1999;117:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Clark JC, Buckingham PD. The preparation and storage of carbon-11 labelled gases for clinical use. Int J Appl Radiat Isot. 1971;22:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Shields AF, Graham MM, Kozawa SM, Kozell LB, Link JM, Swenson ER, Spence AM, Bassingthwaighte JB, Krohn KA. Contribution of labeled carbon dioxide to PET imaging of carbon-11-labeled compounds. J Nucl Med. 1992;33:581-584. [PubMed] |
| 41. | Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | GOLDFARB S, SINGER EJ, POPPER H. BILIARY DUCTULES AND BILE SECRETION. J Lab Clin Med. 1963;62:608-615. [PubMed] |
| 43. | Kountouras J, McKavanagh S, Burmicky M, Billing BH. The effect of secretin on bile flow and bile acid and bilirubin excretion following relief of prolonged bile duct obstruction in the rat. J Hepatol. 1987;4:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Lesage G, Glaser SS, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989;257:G124-G133. [PubMed] |
| 47. | Knuchel J, Krähenbühl S, Zimmermann A, Reichen J. Effect of secretin on bile formation in rats with cirrhosis of the liver: structure-function relationship. Gastroenterology. 1989;97:950-957. [PubMed] |
| 48. | Alpini G, Elias I, Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Francis H, Lasater J, Richards M, LeSage GD. gamma-Interferon inhibits secretin-induced choleresis and cholangiocyte proliferation in a murine model of cirrhosis. J Hepatol. 1997;27:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767-G775. [PubMed] |
| 50. | LeSagE G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | LeSage GD, Glaser SS, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol. 1999;276:G1289-G1301. [PubMed] |
| 52. | LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, Phinizy JL, Baiocchi L, Francis H. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Blanco PG, Zaman MM, Junaidi O, Sheth S, Yantiss RK, Nasser IA, Freedman SD. Induction of colitis in cftr-/- mice results in bile duct injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G491-G496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 56. | Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 58. | Müller M, Roelofsen H, Jansen PL. Secretion of organic anions by hepatocytes: involvement of homologues of the multidrug resistance protein. Semin Liver Dis. 1996;16:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | MILLER LL, BLY CG, WATSON ML, BALE WF. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951;94:431-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 609] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 60. | Beije B, Jenssen D, Arrhenius E, Zetterqvist MA. Isolated liver perfusion--a tool in mutagenicity testing for the evaluation of carcinogens. Chem Biol Interact. 1979;27:41-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Demisch K, Ammedick U, Staib W. Testosterone metabolism in the isolated perfused human foetal liver. Horm Metab Res. 1969;1:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 62. | KRUHØFFER P, MUNTZ JA. Carbohydrate metabolism of the isolated, perfused cat liver as studied by labelled glucose and fructose. Acta Physiol Scand. 1954;30:258-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | BENHAMOU JP, AFIFI FH, LOVERDO A, FAUVERT R. [Fixation of colloidal radioactive gold by isolated perfused rabbit liver. I. Measurement of the efficacy of purification]. C R Seances Soc Biol Fil. 1957;151:442-444. [PubMed] |
| 64. | AXELROD LR, MILLER LL. The metabolism of hydrocortisone in the isolated perfused dog liver. Arch Biochem Biophys. 1956;60:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | ANDREWS WH, BRITTON HG, HUGGETT AS. Fructose metabolism in the isolated perfused liver of the foetal and new-born sheep. J Physiol. 1960;153:199-208. [PubMed] |
| 66. | MARTINIS AJ, GOLDSWORTHY PD, JONES TW, NYHUS LM, DEVITO RV, VOLWILER W, HARKINS HN. Studies of hepatic physiology in the isolated, perfused calf liver. Surg Forum. 1958;9:489-493. [PubMed] |
| 67. | EISEMAN B, MOORE TC, NORMELL L. HISTAMINE METABOLISM IN THE ISOLATED PERFUSED PIG LIVER. Surg Gynecol Obstet. 1964;118:69-74. [PubMed] |
| 68. | Sparks JW, Lynch A, Chez RA, Glinsmann WH. Glycogen regulation in isolated perfused near term monkey liver. Pediatr Res. 1976;10:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Gores GJ, Kost LJ, LaRusso NF. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986;6:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Reichen J, Paumgartner G. Kinetics of taurocholate uptake by the perfused rat liver. Gastroenterology. 1975;68:132-136. [PubMed] |
| 71. | Wanless IR. Physioanatomic considerations. Shiff’s diseases of the liver, New York: Lippincott-Raven 1999; 3-37. |
| 72. | Ahmad AB, Bennett PN, Rowland M. Influence of route of hepatic administration on drug availability. J Pharmacol Exp Ther. 1984;230:718-725. [PubMed] |
| 73. | Zimmermann T, Gardemann A, Machnik G, Dargel R, Jungermann K. Metabolic and hemodynamic responses of bivascularly perfused rat liver to nerve stimulation, noradrenaline, acetylcholine and glucagon in thioacetamide-induced micronodular cirrhosis. Hepatology. 1992;15:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Imamura T, Fujimoto JM. Transit patterns of marker compounds given by segmented retrograde intrabiliary injection (SRII) in the isolated in situ perfused rat liver. J Pharmacol Exp Ther. 1980;215:110-115. [PubMed] |
| 77. | Meier PJ, Sztul ES, Reuben A, Boyer JL. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984;98:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 321] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Meier PJ, Knickelbein R, Moseley RH, Dobbins JW, Boyer JL. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985;75:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Graf J, Gautam A, Boyer JL. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984;81:6516-6520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Benedetti A, Marucci L, Bassotti C, Mancini R, Contucci S, Jezequel AM, Orlandi F. Tubulovesicular transcytotic pathway in rat biliary epithelium: a study in perfused liver and in isolated intrahepatic bile duct. Hepatology. 1993;18:422-432. [PubMed] |
| 81. | Mennone A, Alvaro D, Cho W, Boyer JL. Isolation of small polarized bile duct units. Proc Natl Acad Sci U S A. 1995;92:6527-6531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Cho WK, Mennone A, Boyer JL. Isolation of functional polarized bile duct units from mouse liver. Am J Physiol Gastrointest Liver Physiol. 2001;280:G241-G246. [PubMed] |
| 83. | Boyer JL. Isolated hepatocyte couplets and bile duct units--novel preparations for the in vitro study of bile secretory function. Cell Biol Toxicol. 1997;13:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Gautam A, Ng OC, Strazzabosco M, Boyer JL. Quantitative assessment of canalicular bile formation in isolated hepatocyte couplets using microscopic optical planimetry. J Clin Invest. 1989;83:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Gall JA, Bhathal PS. The isolation of intrahepatic biliary epithelial cells from normal rat livers. Cell Biol Int Rep. 1985;9:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Parola M, Cheeseman KH, Biocca ME, Dianzani MU, Slater TF. Isolation and characterization of biliary epithelial cells from normal rat liver. J Hepatol. 1988;6:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Kumar U, Jordan TW. Isolation and culture of biliary epithelial cells from the biliary tract fraction of normal rats. Liver. 1986;6:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Grisham JW. Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem. 1983;53-54:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236-1247. [PubMed] |
| 90. | Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Cho WK, Siegrist VJ, Zinzow W. Impaired regulatory volume decrease in freshly isolated cholangiocytes from cystic fibrosis mice: implications for cystic fibrosis transmembrane conductance regulator effect on potassium conductance. J Biol Chem. 2004;279:14610-14618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Spirlì C, Fiorotto R, Song L, Santos-Sacchi J, Okolicsanyi L, Masier S, Rocchi L, Vairetti MP, De Bernard M, Melero S. Glibenclamide stimulates fluid secretion in rodent cholangiocytes through a cystic fibrosis transmembrane conductance regulator-independent mechanism. Gastroenterology. 2005;129:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Nozaki I, Lunz JG 3rd, Specht S, Park JI, Giraud AS, Murase N, Demetris AJ. Regulation and function of trefoil factor family 3 expression in the biliary tree. Am J Pathol. 2004;165:1907-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Grubman SA, Fang SL, Mulberg AE, Perrone RD, Rogers LC, Lee DW, Armentano D, Murray SL, Dorkin HL, Cheng SH. Correction of the cystic fibrosis defect by gene complementation in human intrahepatic biliary epithelial cell lines. Gastroenterology. 1995;108:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Zsembery A, Jessner W, Sitter G, Spirlí C, Strazzabosco M, Graf J. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3- secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303-313. [PubMed] |
| 97. | Salter KD, Roman RM, LaRusso NR, Fitz JG, Doctor RB. Modified culture conditions enhance expression of differentiated phenotypic properties of normal rat cholangiocytes. Lab Invest. 2000;80:1775-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Joplin R, Strain AJ, Neuberger JM. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell Dev Biol. 1989;25:1189-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Joplin R, Strain AJ, Neuberger JM. Biliary epithelial cells from the liver of patients with primary biliary cirrhosis: isolation, characterization, and short-term culture. J Pathol. 1990;162:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Joplin R, Hishida T, Tsubouchi H, Daikuhara Y, Ayres R, Neuberger JM, Strain AJ. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest. 1992;90:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Ishida Y, Smith S, Wallace L, Sadamoto T, Okamoto M, Auth M, Strazzabosco M, Fabris L, Medina J, Prieto J. Ductular morphogenesis and functional polarization of normal human biliary epithelial cells in three-dimensional culture. J Hepatol. 2001;35:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1714] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 103. | Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982;95:189-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 809] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 104. | Graber ML, DiLillo DC, Friedman BL, Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986;156:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Strazzabosco M, Boyer JL. Ion Transporters that regulate intracellular pH and secretion in bile duct epithelial cells. Biliary and pancreatic ductal epithelia: Pathobiology and pathophysiology, New York: Marcel Dekker, Inc 1997; 85-106. |
| 106. | Spirlì C, Granato A, Zsembery K, Anglani F, Okolicsányi L, LaRusso NF, Crepaldi G, Strazzabosco M. Functional polarity of Na+/H+ and Cl-/HCO3- exchangers in a rat cholangiocyte cell line. Am J Physiol. 1998;275:G1236-G1245. [PubMed] |
| 107. | Chaillet JR, Amsler K, Boron WF. Optical measurements of intracellular pH in single LLC-PK1 cells: demonstration of Cl-HCO3 exchange. Proc Natl Acad Sci U S A. 1986;83:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28:160-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 109. | Masyuk TV, Ritman EL, LaRusso NF. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. Am J Pathol. 2001;158:2079-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Grisham JW, Nopanitaya W, Compagno J, Nägel AE. Scanning electron microscopy of normal rat liver: the surface structure of its cells and tissue components. Am J Anat. 1975;144:295-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Ishii M, Vroman B, LaRusso NF. Morphologic demonstration of receptor-mediated endocytosis of epidermal growth factor by isolated bile duct epithelial cells. Gastroenterology. 1990;98:1284-1291. [PubMed] |
| 112. | Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper S, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413-20419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | McCaffery JM, Farquhar MG. Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol. 1995;257:259-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Cullen BR. Derivation and function of small interfering RNAs and microRNAs. Virus Res. 2004;102:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Splinter PL, Masyuk AI, LaRusso NF. Specific inhibition of AQP1 water channels in isolated rat intrahepatic bile duct units by small interfering RNAs. J Biol Chem. 2003;278:6268-6274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | Obama K, Ura K, Satoh S, Nakamura Y, Furukawa Y. Up-regulation of PSF2, a member of the GINS multiprotein complex, in intrahepatic cholangiocarcinoma. Oncol Rep. 2005;14:701-706. [PubMed] |
| 117. | Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 118. | Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046-10050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 673] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 119. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 120. | Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 121. | Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 122. | Erlinger S. Physiology of bile secretion and enterohepatic circulation. Physiology of the gastrointestinal tract, New York: Raven Press 1987; 1557-1580. |
| 123. | Meier PJ, Stieger B. Molecular Mechanisms in Bile Formation. News Physiol Sci. 2000;15:89-93. [PubMed] |
| 124. | Benedetti A, Strazzabosco M, Corasanti JG, Haddad P, Graf J, Boyer JL. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991;261:G512-G522. [PubMed] |
| 125. | Boyer JL, Graf J, Meier PJ. Hepatic transport systems regulating pHi, cell volume, and bile secretion. Annu Rev Physiol. 1992;54:415-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 126. | Benedetti A, Strazzabosco M, Ng OC, Boyer JL. Regulation of activity and apical targeting of the Cl-/HCO3- exchanger in rat hepatocytes. Proc Natl Acad Sci U S A. 1994;91:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 127. | Strazzabosco M, Boyer JL. Regulation of intracellular pH in the hepatocyte. Mechanisms and physiological implications. J Hepatol. 1996;24:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Gradilone SA, García F, Huebert RC, Tietz PS, Larocca MC, Kierbel A, Carreras FI, Larusso NF, Marinelli RA. Glucagon induces the plasma membrane insertion of functional aquaporin-8 water channels in isolated rat hepatocytes. Hepatology. 2003;37:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Boyer JL, Bloomer JR. Canalicular bile secretion in man. Studies utilizing the biliary clearance of (14C)mannitol. J Clin Invest. 1974;54:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 78] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Prandi D. Canalicular bile production in man. Eur J Clin Invest. 1975;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 131. | Berthelot P, Erlinger S, Dhumeaux D, Preaux AM. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970;219:809-813. [PubMed] |
| 132. | Boyer JL, Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non-bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology. 1970;59:853-859. [PubMed] |
| 133. | Erlinger S, Dhumeaux D, Berthelot P, Dumont M. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol. 1970;219:416-422. [PubMed] |
| 134. | Lenzen R, Hruby VJ, Tavoloni N. mechanism of glucagon choleresis in guinea pigs. Am J Physiol. 1990;259:G736-G744. [PubMed] |
| 135. | Alvaro D, Della Guardia P, Bini A, Gigliozzi A, Furfaro S, La Rosa T, Piat C, Capocaccia L. Effect of glucagon on intracellular pH regulation in isolated rat hepatocyte couplets. J Clin Invest. 1995;96:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | García F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, LaRusso NF, Marinelli RA. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem. 2001;276:12147-12152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 137. | Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710-22717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Gradilone SA, Tietz PS, Splinter PL, Marinelli RA, LaRusso NF. Expression and subcellular localization of aquaporin water channels in the polarized hepatocyte cell line, WIF-B. BMC Physiol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 139. | Bruck R, Benedetti A, Strazzabosco M, Boyer JL. Intracellular alkalinization stimulates bile flow and vesicular-mediated exocytosis in IPRL. Am J Physiol. 1993;265:G347-G353. [PubMed] |
| 140. | Tietz P, Jefferson J, Pagano R, Larusso NF. Membrane microdomains in hepatocytes: potential target areas for proteins involved in canalicular bile secretion. J Lipid Res. 2005;46:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol. 1999;276:G280-G286. [PubMed] |
| 142. | Tietz PS, McNiven MA, Splinter PL, Huang BQ, Larusso NF. Cytoskeletal and motor proteins facilitate trafficking of AQP1-containing vesicles in cholangiocytes. Biol Cell. 2006;98:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Martínez-Ansó E, Castillo JE, Díez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 144. | Aranda V, Martínez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020-12025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 268] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 146. | Roman RM, Bodily KO, Wang Y, Raymond JR, Fitz JG. Activation of protein kinase Calpha couples cell volume to membrane Cl- permeability in HTC hepatoma and Mz-ChA-1 cholangiocarcinoma cells. Hepatology. 1998;28:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 147. | Schöfl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Mühlen A, Mix H, Cornberg M, Böker KH, Manns MP. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164-G172. [PubMed] |
| 148. | Feranchak AP, Fitz JG, Roman RM. Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol. 2000;33:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 149. | Renner EL, Lake JR, Scharschmidt BF, Zimmerli B, Meier PJ. Rat hepatocytes exhibit basolateral Na+/HCO3- cotransport. J Clin Invest. 1989;83:1225-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Gleeson D, Smith ND, Boyer JL. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989;84:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Fitz JG, Persico M, Scharschmidt BF. Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol. 1989;256:G491-G500. [PubMed] |
| 152. | Buanes T, Grotmol T, Veel T, Landsverk T, Ridderstråle Y, Raeder MG. Importance of carbonic anhydrase for canalicular and ductular choleresis in the pig. Acta Physiol Scand. 1988;133:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 153. | Kivelä AJ, Kivelä J, Saarnio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol. 2005;11:155-163. [PubMed] |
| 154. | Henry RP. Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol. 1996;58:523-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 211] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 155. | Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992;267:9331-9339. [PubMed] |
| 156. | Moseley RH, Meier PJ, Aronson PS, Boyer JL. Na-H exchange in rat liver basolateral but not canalicular plasma membrane vesicles. Am J Physiol. 1986;250:G35-G43. [PubMed] |
| 157. | Grinstein S, Woodside M, Waddell TK, Downey GP, Orlowski J, Pouyssegur J, Wong DC, Foskett JK. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: assessment of effects on intracellular pH. EMBO J. 1993;12:5209-5218. [PubMed] |
| 158. | Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol. 1998;275:F510-F517. [PubMed] |
| 159. | Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL. Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G247-G254. [PubMed] |
| 160. | Fitz JG, Lidofsky SD, Xie MH, Scharschmidt BF. Transmembrane electrical potential difference regulates Na+/HCO3- cotransport and intracellular pH in hepatocytes. Proc Natl Acad Sci U S A. 1992;89:4197-4201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 161. | Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447:495-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 162. | Kurtz I, Petrasek D, Tatishchev S. Molecular mechanisms of electrogenic sodium bicarbonate cotransport: structural and equilibrium thermodynamic considerations. J Membr Biol. 2004;197:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 163. | Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689-17695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 164. | Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol. 2004;287:C781-C789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 165. | Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000;1493:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 166. | Kaminski DL, Dorighi J, Jellinek M. Effect of electrical vagal stimulation on canine hepatic bile flow. Am J Physiol. 1974;227:487-493. [PubMed] |
| 167. | Cucchiaro G, Branum GD, Farouk M, Mansour G, Kuhn CM, Anthony DC, Meyers WC. The effects of liver denervation on the regulation of hepatic biliary secretion. Transplantation. 1992;54:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Strazzabosco M. New insights into cholangiocyte physiology. J Hepatol. 1997;27:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 169. | Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 170. | Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 171. | Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 172. | Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 173. | Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, LaRusso NF. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci U S A. 2000;97:11092-11097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Strazzabosco M, Mennone A, Boyer JL. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991;87:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 175. | Alvaro D, Cho WK, Mennone A, Boyer JL. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993;92:1314-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 176. | Strazzabosco M, Joplin R, Zsembery A, Wallace L, Spirlì C, Fabris L, Granato A, Rossanese A, Poci C, Neuberger JM. Na(+)-dependent and -independent Cl-/HCO-3 exchange mediate cellular HCO3- transport in cultured human intrahepatic bile duct cells. Hepatology. 1997;25:976-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 177. | Ueno Y, Ishii M, Takahashi S, Igarashi T, Toyota T, LaRusso NF. Different susceptibility of mice to immune-mediated cholangitis induced by immunization with carbonic anhydrase II. Lab Invest. 1998;78:629-637. [PubMed] |
| 178. | Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060-G1070. [PubMed] |
| 179. | Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na(+)-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358-8363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 180. | Villanger O, Veel T, Raeder MG. Secretin causes H+ secretion from intrahepatic bile ductules by vacuolar-type H(+)-ATPase. Am J Physiol. 1993;265:G719-G724. [PubMed] |
| 181. | Burckhardt G, Di Sole F, Helmle-Kolb C. The Na+/H+ exchanger gene family. J Nephrol. 2002;15 Suppl 5:S3-21. [PubMed] |
| 182. | Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781-12789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 183. | Alvaro D, Mennone A, Boyer JL. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3- exchange in cholangiocytes. Am J Physiol. 1997;273:G303-G313. [PubMed] |
| 184. | Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 185. | Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 186. | Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl-/HCO3- exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1093-G1103. [PubMed] |
| 187. | Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl- channel regulated by intracellular pH. J Biol Chem. 2005;280:6463-6470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 188. | Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993;91:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 189. | Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857-1864. [PubMed] |
| 190. | Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990;259:C752-C761. [PubMed] |
| 191. | Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3- conductance through CFTR in human airway epithelia. Am J Physiol. 1997;272:L752-L761. [PubMed] |
| 192. | Choi JY, Lee MG, Ko S, Muallem S. Cl(-)-dependent HCO3- transport by cystic fibrosis transmembrane conductance regulator. JOP. 2001;2:243-246. [PubMed] |
| 193. | Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340-5344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 324] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 194. | Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 195. | Spirlì C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, Larusso NF, Sonzogni A, Okolicsanyi L, Strazzabosco M. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003;124:737-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 196. | Basavappa S, Middleton J, Mangel AW, McGill JM, Cohn JA, Fitz JG. Cl- and K+ transport in human biliary cell lines. Gastroenterology. 1993;104:1796-1805. [PubMed] |
| 197. | McGill JM, Basavappa S, Mangel AW, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology. 1994;107:236-243. [PubMed] |
| 198. | Schlenker T, Fitz JG. Ca(2+)-activated C1- channels in a human biliary cell line: regulation by Ca2+/calmodulin-dependent protein kinase. Am J Physiol. 1996;271:G304-G310. [PubMed] |
| 199. | Clarke LL, Harline MC, Gawenis LR, Walker NM, Turner JT, Weisman GA. Extracellular UTP stimulates electrogenic bicarbonate secretion across CFTR knockout gallbladder epithelium. Am J Physiol Gastrointest Liver Physiol. 2000;279:G132-G138. [PubMed] |
| 200. | McGill JM, Yen MS, Basavappa S, Mangel AW, Kwiatkowski AP. ATP-activated chloride permeability in biliary epithelial cells is regulated by calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;208:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 201. | Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl- secretion in cultured human and rat biliary epithelial cells. Am J Physiol. 1999;276:G1391-G1400. [PubMed] |
| 202. | Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology. 2004;127:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 203. | McGill JM, Basavappa S, Fitz JG. Characterization of high-conductance anion channels in rat bile duct epithelial cells. Am J Physiol. 1992;262:G703-G710. [PubMed] |
| 204. | Singh SK, Mennone A, Gigliozzi A, Fraioli F, Boyer JL. Cl(-)-dependent secretory mechanisms in isolated rat bile duct epithelial units. Am J Physiol Gastrointest Liver Physiol. 2001;281:G438-G446. [PubMed] |
| 205. | Geck P, Pfeiffer B. Na+ + K+ + 2Cl- cotransport in animal cells--its role in volume regulation. Ann N Y Acad Sci. 1985;456:166-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 206. | Panet R, Markus M, Atlan H. Bumetanide and furosemide inhibited vascular endothelial cell proliferation. J Cell Physiol. 1994;158:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 207. | Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211-276. [PubMed] |
| 208. | Scoazec JY, Bringuier AF, Medina JF, Martínez-Ansó E, Veissiere D, Feldmann G, Housset C. The plasma membrane polarity of human biliary epithelial cells: in situ immunohistochemical analysis and functional implications. J Hepatol. 1997;26:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 209. | Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1991;254:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 210. | Rakowski RF, Gadsby DC, De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989;93:903-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 211. | McRoberts JA, Beuerlein G, Dharmsathaphorn K. Cyclic AMP and Ca2+-activated K+ transport in a human colonic epithelial cell line. J Biol Chem. 1985;260:14163-14172. [PubMed] |
| 212. | Roman R, Feranchak AP, Troetsch M, Dunkelberg JC, Kilic G, Schlenker T, Schaack J, Fitz JG. Molecular characterization of volume-sensitive SK(Ca) channels in human liver cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G116-G122. [PubMed] |
| 213. | Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem. 1997;272:23195-23200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 214. | Marinelli RA, Pham LD, Tietz PS, LaRusso NF. Expression of aquaporin-4 water channels in rat cholangiocytes. Hepatology. 2000;31:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 215. | Splinter PL, Masyuk AI, Marinelli RA, LaRusso NF. AQP4 transfected into mouse cholangiocytes promotes water transport in biliary epithelia. Hepatology. 2004;39:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 216. | Talbot NC, Garrett WM, Caperna TJ. Analysis of the expression of aquaporin-1 and aquaporin-9 in pig liver tissue: comparison with rat liver tissue. Cells Tissues Organs. 2003;174:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 217. | Still EU, McBean JW, Ries FA. Studies on the physiology of secretin. IV. Effect on the secretion of bile. Ame J Physiol. 1931;99:94. |
| 218. | Mellanby JM, Suffolk FS. Quantitative investigation into enterohepatic circulation of bile salts in the cat. Proc R Soc. 1938;126:287. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 219. | GROSSMAN MI, JANOWITZ HD. The effect of secretin on bile formation in man. Gastroenterology. 1949;12:133-138. [PubMed] |
| 220. | JORPES JE, MUTT V, MAGNUSSON S, STEELE BB. Amino acid composition and N-terminal amino acid sequence of porcine secretin. Biochem Biophys Res Commun. 1962;9:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 221. | Mutt V, Jorpes JE, Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970;15:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 117] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 222. | Ondetti MA, Narayanan VL, von Saltza M, Sheehan JT, Sabo EF, Bodanszky M. The synthesis of secretin. 3. The fragment-condensation approach. J Am Chem Soc. 1968;90:4711-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 223. | Joffe SN, Bloom SR, Polak JM, Welbourn RB. Proceedings: Release of secretin for S cells of porcine duodenum and jejunum by acid. Gut. 1975;16:398. [PubMed] |
| 224. | Polak JM, Bloom SR, Kuzio M, Brown JC, Pearse AG. Cellular localization of gastric inhibitory polypeptide in the duodenum and jejunum. Gut. 1973;14:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 225. | Kim MS, Lee KY, Chey WY. Plasma secretin concentrations in fasting and postprandial states in dog. Am J Physiol. 1979;236:E539-E544. [PubMed] |
| 226. | Sun G, Lee KY, Chang TM, Chey WY. Effect of pancreatic juice diversion on secretin release in rats. Gastroenterology. 1989;96:1173-1179. [PubMed] |
| 227. | Shiratori K, Jo YH, Lee KY, Chang TM, Chey WY. Effect of pancreatic juice and trypsin on oleic acid-stimulated pancreatic secretion and plasma secretin in dogs. Gastroenterology. 1989;96:1330-1336. [PubMed] |
| 228. | Li P, Lee KY, Chang TM, Chey WY. Mechanism of acid-induced release of secretin in rats. Presence of a secretin-releasing peptide. J Clin Invest. 1990;86:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 229. | Li P, Song Y, Lee KY, Chang TM, Chey WY. A secretin releasing peptide exists in dog pancreatic juice. Life Sci. 2000;66:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 230. | Li JP, Lee KY, Chang TM, Chey WY. MEK inhibits secretin release and pancreatic secretion: roles of secretin-releasing peptide and somatostatin. Am J Physiol Gastrointest Liver Physiol. 2001;280:G890-G896. [PubMed] |
| 231. | Chey WY, Kim MS, Lee KY, Chang TM. Secretin is an enterogastrone in the dog. Am J Physiol. 1981;240:G239-G244. [PubMed] |
| 232. | Jin HO, Lee KY, Chang TM, Chey WY, Dubois A. Secretin: a physiological regulator of gastric emptying and acid output in dogs. Am J Physiol. 1994;267:G702-G708. [PubMed] |
| 233. | Chung I, Li P, Lee K, Chang T, Chey WY. Dual inhibitory mechanism of secretin action on acid secretion in totally isolated, vascularly perfused rat stomach. Gastroenterology. 1994;107:1751-1758. [PubMed] |
| 234. | Shimizu K, Li P, Lee KY, Chang TM, Chey WY. The mechanism of inhibitory action of secretin on gastric acid secretion in conscious rats. J Physiol. 1995;488:501-508. [PubMed] |
| 235. | Raybould HE, Holzer H. Secretin inhibits gastric emptying in rats via a capsaicin-sensitive vagal afferent pathway. Eur J Pharmacol. 1993;250:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 236. | Lu Y, Owyang C. Secretin at physiological doses inhibits gastric motility via a vagal afferent pathway. Am J Physiol. 1995;268:G1012-G1016. [PubMed] |
| 237. | Holtmann MH, Ganguli S, Hadac EM, Dolu V, Miller LJ. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J Biol Chem. 1996;271:14944-14949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 238. | Pang RT, Ng SS, Cheng CH, Holtmann MH, Miller LJ, Chow BK. Role of N-linked glycosylation on the function and expression of the human secretin receptor. Endocrinology. 1999;140:5102-5111. [PubMed] [DOI] [Full Text] |
| 239. | Gardner JD, Jensen RT. Regulation of pancreatic enzyme secretion in vitro. Physiology of the gastrointestinal tract. Vol. 2. New York: Raven Press 1981; 831-837. |
| 240. | Farouk M, Vigna SR, McVey DC, Meyers WC. Localization and characterization of secretin binding sites expressed by rat bile duct epithelium. Gastroenterology. 1992;102:963-968. [PubMed] |
| 241. | Farouk M, Vigna SR, Haebig JE, Gettys TW, McVey DC, Chari R, Pruthi RS, Meyers WC. Secretin receptors in a new preparation of plasma membranes from intrahepatic biliary epithelium. J Surg Res. 1993;54:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 242. | Lenzen R, Elster J, Behrend C, Hampel KE, Bechstein WO, Neuhaus P. Bile acid-independent bile flow is differently regulated by glucagon and secretin in humans after orthotopic liver transplantation. Hepatology. 1997;26:1272-1281. [PubMed] |
| 243. | Levine RA, Hall RC. Cyclic AMP in secretin choleresis. Evidence for a regulatory role in man and baboons but not in dogs. Gastroenterology. 1976;70:537-544. [PubMed] |
| 244. | McGill JM, Basavappa S, Gettys TW, Fitz JG. Secretin activates Cl- channels in bile duct epithelial cells through a cAMP-dependent mechanism. Am J Physiol. 1994;266:G731-G736. [PubMed] |
| 245. | Alpini G, Ulrich CD 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol. 1994;266:G922-G928. [PubMed] |
| 246. | Tietz PS, Hadac EM, Miller LJ, LaRusso NF. Upregulation of secretin receptors on cholangiocytes after bile duct ligation. Regul Pept. 2001;97:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 247. | Mutt V. Secretin: isolation, structure and function. Gastrointestinal hormones. New York: Raven Press 1980; 85-126. |
| 248. | Hirata K, Nathanson MH. Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology. 2001;121:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 249. | PREISIG R, COOPER HL, WHEELER HO. The relationship between taurocholate secretion rate and bile production in the unanesthetized dog during cholinergic blockade and during secretin administration. J Clin Invest. 1962;41:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 250. | Alpini G, Glaser S, Francis H, Marzioni M, Venter J, LeSage G. Bile acid interaction with cholangiocytes. The Pathophysiology of the Biliary Epithelia. Georgetown, TX: Landes Bioscience 2004; 112-126. |
| 251. | Bijvelds MJ, Jorna H, Verkade HJ, Bot AG, Hofmann F, Agellon LB, Sinaasappel M, de Jonge HR. Activation of CFTR by ASBT-mediated bile salt absorption. Am J Physiol Gastrointest Liver Physiol. 2005;289:G870-G879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 252. | Dumont M, Erlinger S, Uchman S. Hypercholeresis induced by ursodeoxycholic acid and 7-ketolithocholic acid in the rat: possible role of bicarbonate transport. Gastroenterology. 1980;79:82-89. [PubMed] |
| 253. | Yoon YB, Hagey LR, Hofmann AF, Gurantz D, Michelotti EL, Steinbach JH. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837-852. [PubMed] |
| 254. | Palmer KR, Gurantz D, Hofmann AF, Clayton LM, Hagey LR, Cecchetti S. Hypercholeresis induced by norchenodeoxycholate in biliary fistula rodent. Am J Physiol. 1987;252:G219-G228. [PubMed] |
| 255. | Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol. 1996;271:G86-G96. [PubMed] |
| 256. | Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser SS. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 257. | Mann R, Bhathal PS, Bell C. Aminergic innervation of the gall bladder in man and dog. Clin Auton Res. 1991;1:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 258. | Mann R, Bhathal PS, Bell C. Sympathetic innervation of the liver in man and dog: an immunohistochemical study. Clin Auton Res. 1991;1:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 259. | Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683-G694. [PubMed] |
| 260. | Kanno N, Lesage G, Phinizy JL, Glaser S, Francis H, Alpini G. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 261. | LeSage GD, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 262. | Kaminski DL, Deshpande YG. Effect of somatostatin and bombesin on secretin-stimulated ductular bile flow in dogs. Gastroenterology. 1983;85:1239-1247. [PubMed] |
| 263. | Kortz WJ, Nashold JR, Delong E, Meyers WC. Effects of bombesin on fasting bile formation. Ann Surg. 1986;203:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 264. | Cho WK, Mennone A, Rydberg SA, Boyer JL. Bombesin stimulates bicarbonate secretion from rat cholangiocytes: implications for neural regulation of bile secretion. Gastroenterology. 1997;113:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 265. | Cho WK, Mennone A, Boyer JL. Intracellular pH regulation in bombesin-stimulated secretion in isolated bile duct units from rat liver. Am J Physiol. 1998;275:G1028-G1036. [PubMed] |
| 266. | Cho WK, Boyer JL. Characterization of ion transport mechanisms involved in bombesin-stimulated biliary secretion in rat cholangiocytes. J Hepatol. 1999;30:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 267. | Nyberg B, Einarsson K, Sonnenfeld T. Evidence that vasoactive intestinal peptide induces ductular secretion of bile in humans. Gastroenterology. 1989;96:920-924. [PubMed] |
| 268. | Nyberg B, Sonnenfeld T, Einarsson K. Vasoactive intestinal peptide and secretin: effects of combined and separate intravenous infusions on bile secretion in man. Scand J Gastroenterol. 1991;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 269. | Cho WK, Boyer JL. Vasoactive intestinal polypeptide is a potent regulator of bile secretion from rat cholangiocytes. Gastroenterology. 1999;117:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 270. | Harada E, Niiyama M, Syuto B. Hepatic bile and pancreatic exocrine secretions evoked by gastrointestinal peptides in sheep. Comp Biochem Physiol A Comp Physiol. 1986;85:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 271. | Makhlouf GM, Yau WM, Zfass AM, Said SI, Bodanszky M. Comparative effects of synthetic and natural vasoactive intestinal peptide on pancreatic and biliary secretion and on glucose and insulin blood levels in the dog. Scand J Gastroenterol. 1978;13:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 272. | Tietz PS, Alpini G, Pham LD, Larusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol. 1995;269:G110-G118. [PubMed] |
| 273. | Gong AY, Tietz PS, Muff MA, Splinter PL, Huebert RC, Strowski MZ, Chen XM, LaRusso NF. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol. 2003;284:C1205-C1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 274. | Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol. 1997;273:G1061-G1070. [PubMed] |
| 275. | Lesage GD, Marucci L, Alvaro D, Glaser SS, Benedetti A, Marzioni M, Patel T, Francis H, Phinizy JL, Alpini G. Insulin inhibits secretin-induced ductal secretion by activation of PKC alpha and inhibition of PKA activity. Hepatology. 2002;36:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 276. | Kilic G, Doctor RB, Fitz JG. Insulin stimulates membrane conductance in a liver cell line: evidence for insertion of ion channels through a phosphoinositide 3-kinase-dependent mechanism. J Biol Chem. 2001;276:26762-26768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 277. | Mabuchi M, Kawamura I, Fushimi M, Takeshita S, Takakura S, Hirosumi J, Mutoh S. Choleretic actions of insulin-like growth factor-I, prednisolone, and ursodeoxycholic acid in rats. Dig Dis Sci. 2003;48:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 278. | Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 279. | Alvaro D, Gigliozzi A, Marucci L, Alpini G, Barbaro B, Monterubbianesi R, Minetola L, Mancino MG, Medina JF, Attili AF. Corticosteroids modulate the secretory processes of the rat intrahepatic biliary epithelium. Gastroenterology. 2002;122:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 280. | Alvaro D, Gigliozzi A, Piat C, Carli L, Fraioli F, Romeo R, Francia C, Attili AF, Capocaccia L. Inhibition of biliary bicarbonate secretion in ethinyl estradiol-induced cholestasis is not associated with impaired activity of the Cl-/HCO-3 exchanger in the rat. J Hepatol. 1997;26:146-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 281. | Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 282. | Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis. 2002;22:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 283. | Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol. 2004;555:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 284. | Dranoff JA, Nathanson MH. It's swell to have ATP in the liver. J Hepatol. 2000;33:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 285. | Dranoff JA. Purinergic regulation of bile ductular secretion. The Pathophysiology of the Biliary Epithelia. Georgetown, TX: Landes Bioscience 2004; 96-104. |
| 286. | Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419-F432. [PubMed] |
| 287. | Zsembery A, Spirlì C, Granato A, LaRusso NF, Okolicsanyi L, Crepaldi G, Strazzabosco M. Purinergic regulation of acid/base transport in human and rat biliary epithelial cell lines. Hepatology. 1998;28:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 288. | Nguyen TD, Meichle S, Kim US, Wong T, Moody MW. P2Y(11), a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G795-G804. [PubMed] |
| 289. | McGill JM, Yen MS, Cummings OW, Alpini G, LeSage G, Pollok KE, Miller B, Engle SK, Stansfield AP. Interleukin-5 inhibition of biliary cell chloride currents and bile flow. Am J Physiol Gastrointest Liver Physiol. 2001;280:G738-G745. [PubMed] |
| 290. | Spirlì C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, Di Virgilio F, Okolicsanyi L, Casagrande F, Strazzabosco M. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |