Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3471
Revised: February 6, 2006
Accepted: February 18, 2006
Published online: June 14, 2006
Cholangiocytes, the epithelial cells lining the biliary ducts, are the target cells in several liver diseases. Cholangiopathies and cholangiocarcinoma generate interest in many scientists since the genesis. The developing mechanisms, and the therapeutic tools of these diseases are still undefined. Several studies demonstrate that many hormones, neuropeptides and neurotransmitters regulate malignant and non-malignant cholangiocyte pathophysiology in the course of chronic biliary diseases. The aim of this review is to present the findings of several studies published in the recent years that contributed to clarifying the role of nervous and neuroendocrine regulation of the pathophysiologic events associated with cholestasis and cholangiocarcinoma development. This manuscript is organized into two parts. The first part offers an overview of the innervation of the liver and the origin of neuroendocrine hormones, neurotransmitters and neuropeptides affecting cholangiocyte function and metabolism. The first section also reviews the effects played by several neuroendocrine hormones and nervous system on cholangiocyte growth, survival and functional activity in the course of cholestasis. In the second section, we summarize the results of some studies describing the role of nervous system and neuroendocrine hormones in the regulation of malignant cholangiocyte growth.
- Citation: Marzioni M, Fava G, Benedetti A. Nervous and Neuroendocrine regulation of the pathophysiology of cholestasis and of biliary carcinogenesis. World J Gastroenterol 2006; 12(22): 3471-3480
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3471
Cholangiocytes are the epithelial cells that line the intra-hepatic biliary tree. Their physiologic role is to modify the bile of canalicular origin, through a wide array of absorbtive and secretive processes[1,2]. Indeed, although in normal conditions they represent approximately the 4%-5% of the liver mass, they contribute for at least the 10%-30% of the bile flow[1].
Cholangiocytes are the target of chronic diseases, termed cholangiopathies[3,4], that are commonly characterized by dysregulation of the balance between cell growth and survival. In the course of such diseases there is impaired proliferative response to duct injury and increased cell death by apoptosis, that leads to vanishing of bile ducts[3] and functional liver failure at the end stage. Cholangiopathies thus represent a daily challenge for the clinicians, since definitive medical treatments are not available yet. As a consequence, 20% of liver transplants among adults and 50% of those among pediatric patients are due to these disorders[5].
The malignant transformation of cholangiocytes gives origin to cholangiocarcinoma[6]. Cholangiocarcinoma is often diagnosed at late stages; its surgical resection has given very limited success, as response of this neoplasm to conventional chemotherapy[6] is minimal. These features make cholangiocarcinoma a malignancy characterized by a very poor prognosis. In addition, the incidence and prevalence of cholangiocarcinoma are increasing worldwide[7].
Many of the problems that arise in the management of cholangiopathies and of cholangiocarcinoma can at least in part be ascribed to the poor knowledge about their pathophysiology. Very little is in fact known on which factors are able to affect cholangiocyte biology, with particular regard to those endogenous molecules or systems that affect either cholangiocyte proliferation, survival or functional activity[4].
Bile acids are endogenous factors that have been historically investigated more in this regard, and their properties in modulating cholangiocyte biology and cholangiocarcinoma development are now unanimously accepted[8-13]. More recently, several studies demonstrated that many hormones, neuropeptides and neurotransmitters are able to affect malignant and non malignant cho-langiocyte biology as well. This data showed that the biliary epithelium is similar to the gastric or intestinal epithelia, for which the key role played by nerves and neuroendocrine hormones has been known for years[14]. Moreover, it has been shown that in the course of chronic cholestasis, cholangiocytes acquire a neuroendocrine phenotype, that is not proper for these cells in normal conditions[15]. Immunohistochemical studies have also reported that many biliary tract carcinomas (up to the 70% of the cases) show a neuroendocrine differentiation[16,17].
The purpose of this review is therefore to summarize the evidences from the several studies published in the recent years that contributed to clarify the role of nervous and neuroendocrine regulation of the pathophysiologic events associated with cholestasis and cholangiocarcinoma development. In particular, we will focus on how these factors affect cholangiocyte cell biology. We suggest those who might be interested to learn the effects of nerves and neuroendocrine hormones on other liver cell type biology to other reviews recently published on this subject[18-20].
Neuroendocrine hormones are secreted by neuroendocrine cells, that are diffusely present in the whole gastrointestinal tract, in particular the stomach, small bowel[21], as well as pancreas[22]. If these cells are considered the main source of the neuroendocrine hormones, in this review some studies suggest that cholangiocytes them-selves can synthesize some of these peptides in the course of cholestasis.
The liver is innervated by both sympathetic and para-sympathetic nerves, whose fibers are located around the hepatic artery, portal vein, intrahepatic and extrahepatic bile ducts[23,24]. Sympathetic nerves originate from the celiac ganglion, whereas the parasympathetic from the vagus nerve[23,24]. Besides catecholamines and acetylcholine, autonomic fibers that innervate the liver can also release other neurotransmitters, like neuropeptide Y (NPY)[25,26], calcitonin gene related peptide (CGRP), somatostatin, vasoactive intestinal polypeptide (VIP), enkephalin and bombesin[27-30]. Nerve terminations mostly follow the vascular structures of the portal tract, and have been identified around bile ducts and vessels. Many of the above mentioned neurotransmitters have been shown to modulate intrahepatic hemodynamics[31-33].
| Hormone | Receptor | Effect on cholangiocyte biology | Reference |
| Secretin | SR | Stimulates HCO3- secretion through the increase of cAMP/PKA and opening of CFTR | 1, 34-43 |
| Somatostatin | SSTR2 | Counteracts the effect of secretin; stimulates bile absorption | 44, 45 |
| Insulin | IR | Counteracts the effect of secretin | 46 |
| ET-1 | ETA-ETB | Counteracts the effect of secretin; reduces the SR expression | 47 |
| VIP | unspecified | Increases HCO3- and water secretion | 48, 49 |
| Bombesin | unspecified | Increases HCO3- and water secretion | 50 |
| Gastrin | CCK-B/gastrin | Counteracts the effect of secretin; reduces cell proliferation | 40, 51 |
| Estrogens | ERα-ERβ | Stimulate cell proliferation | 52-55 |
| Serotonin | 5HT1A-5HT1B | Synthesized and secreted by cholangiocytes in the course of cholestasis. Counteracts the effect of secretin and reduces cell growth | 58 |
| GH/IGF-1 | GH-R-IGF-1R | Stimulate cell proliferation | 59 |
The close link between cholangiocytes and neuroendocrine hormones has its basis on the early studies that showed the hormone secretin as the most potent regulator of cholangiocyte functional activity[34,35]. In 1988, Alpini et al demonstrated that in a rat model of cholestasis (induced by bile duct ligation, BDL) the infusion of secretin was associated with a marked increase of the bile flow and bicarbonate biliary excretion[35]. After that "landmark" manuscript, a large series of investigations later defined the intracellular mechanisms by which secretin induces such a potent functional stimulus. It is now known that secretin stimulates ductal secretion[1,34-36] by selective interaction with secretin receptors, expressed only by cholangiocytes in rat liver[37]. The interaction of secretin with its own receptors[37] leads to an increase in intracellular cAMP levels[1,36,38-40], activation of PKA[41], opening of CFTR Cl- channels[42] with activation of the Cl-/HCO3- exchanger[1,36,41,43], which leads to secretion of bicarbonate into bile[35].
Other neuroendocrine hormones were then discovered to affect cholangiocyte choleretic activity. One of the first molecules studied was somatostatin. It was demonstrated that cholangiocytes express the SSTR2 receptor; the interaction of somatostatin with this receptor markedly diminished the effect of secretin on the biliary excretion of water and bicarbonate by cholangiocytes in cholestatic conditions[44]. Such an effect of somatostatin was due to the fact that the activation of the SSTR2 receptor prevented the increase of the adenylyl-cyclase elicited by secretin[44]. In later studies conduced in IBDUs isolated from wild type and SSTR2-knock out mice, it was also found that somatostatin not only reduces cholangiocyte choleresis, but it also stimulates ductal bile absorption[45].
Cholangiocytes also express at the apical pole the receptor for insulin[46]. Upon its activation, a marked reduction of the secretin-induced choleresis was observed, both if the hormone was administered in vivo to BDL rats and if microinjected into the lumen of IBDUs isolated from animals with cholestasis. It was observed that the activation of the insulin receptor determined a cascade of intracellular events that resulted in the inhibition of secretin-stimulated cAMP and PKA activity. Such a chain of events seemed to have its core event in the enhancement of the intracellular Ca2+ levels and the consequent activation of the Ca2+-dependent PKCα[46]. An effect similar to the one of insulin has been described for endothelin-1 (ET-1), which interacts with the specific receptors expressed in the biliary epithelium (ETA and ETB) and blunts the secretin-induced choleresis of the BDL rat and reduces the expression of the secretin receptor on cholangiocytes[47].
In contrast to somatostatin and insulin, vasoactive intestinal polypeptide (VIP) and bombesin have been found to be able to enhance cholangiocyte choleresis. Both the hormones induced a potent fluid and bicarbonate excretion in IBDUs, but not in hepatocytes, isolated from normal and cholestatic rats. Interestingly, neither VIP nor bombesin had any significant effect on modulating intracellular cAMP levels[48-50]. Detailed pH studies indicated that the underlying intracellular mechanism, at least for bombesin, is its ability to stimulate the activity of Cl-/HCO3- exchange in association with a counterbalancing secondary activation of electrogenic Na+/HCO3- symport[50].
In more recent studies, increasing evidence regarding the ability of neuroendocrine hormones to affect cholan-giocyte growth, and functional activity, hvee been reported.
If the infusion of gastrin to BDL rats is associated with the reduction of the choleretic response to secretin by cholangiocytes[40], its chronic administration through an intraperitoneal minipump resulted not only in reduced functional activity, but also in a marked decrease of the bile duct mass[51]. Cholangiocytes indeed express the CCK-B/gastrin receptors[51], which, upon activation, elicit the intracellular Ca2+ release, the increase of IP3 levels, and the membrane translocation (e.g. activation) of the Ca2+-dependent PKCα[51]. In turn, the gastrin-activated PKCα, as above mentioned, is able to interfere with the secretin signaling and modulate the adenyl-cyclase activity, thus reducing the intracellular cAMP levels and PKA activity. These observations, demonstrated that bile acids also activate this intracellular pathway to modulate cholangiocyte growth[8], contribute to elucidate which intracellular signalings sustain the proliferative response of cholangiocytes to cholestasis and by which mechanisms it is possible to modulate them. Moreover, since cAMP/PKA resulted the key molecules implicated in cholangiocyte proliferation, this helped to explain the association between increase of duct mass and enhanced biliary choleresis[8,35].
A major contribution to this field of research has been given by the studies that clarified the effects of estrogens on the biliary epithelium. Both the estrogen receptor (ER) α and β were observed in cholangiocytes[52], their expression being up-regulated after BDL[53]. When cholangiocytes were stimulated in vitro with 17-β-estradiol, their proliferation was markedly increased, as a con-sequence of the ER-dependent activation of Src/Shc/ERK1/2 intracellular pathway[54]. To demonstrate the physiological and pathophysiological relevance of estrogens on cholangiocyte proliferative response to cholestasis, when BDL male rats were treated in vivo with antiestrogens like tamoxifen or Ici 182 780[52] or when BDL female rats were subjected to ovariectomy[53], the growth of the biliary tree was blunted and the biliary epithelium underwent programmed cell death by apoptosis[52,53]. This evidence suggested that estrogens are required for a separative response of the biliary tree to injury. These studies produced in the last few years by Alvaro represent a significant change in the knowledge of the role played by the neuroendocrine system on the biliary cell biology. Indeed, primary biliary cirrhosis (PBC), the most common from of cholangiopathies[4], is much more frequent in women than in men, and has its clinical outcome typically after menopause, when the endogenous estrogen levels suddenly drop[55]. Therefore, these observations by Alvaro seem to provide the biological confirmation of the role of estrogens in the progression of PBC, a role that was, earlier, only hypothesized on the basis of epidemiological data. To further support this concept, Alvaro also demonstrated that the ER expression in cholangiocytes is markedly reduced in late stages of PBC[55]. Altogether, Alvaro’s studies made clear that gaining knowledge on the role of neuroendocrine hormones on cholangiocyte biology might also be an effective strategy to further understand the pathophysiology of the cholangiopathies and thus eventually to design novel therapeutic strategies.
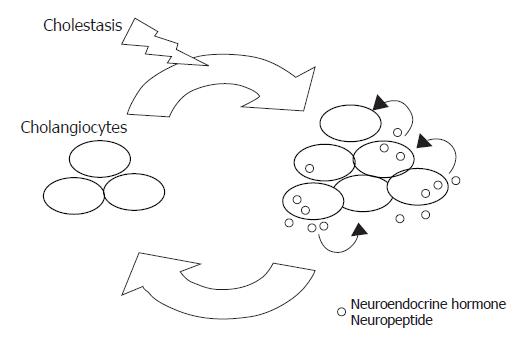
The neuroendocrine hormone serotonin has been hypothesized to be involved in the genesis of certain clinical features of PBC, like fatigue and pruritus[56,57]. Interestingly, it has been recently shown that cholangiocytes express the serotonin 1A and 1B receptors[58]. When they are activated by selective agonists, the proliferation of BDL cholangiocytes are dramatically reduced, both in vivo and in vitro[58], in association with the loss of the response to secretin of the markers of cholangiocyte functional activity[58], like the bile flow, the bicarbonate excretion and the intracellular cAMP levels[34]. Such an effect seems more to be mediated by the cross-talk between the Ca2+ and the cAMP/PKA signalings. If the serotonin 1A and 1B receptors are activated, there is an increase of Ca2+, IP3 and PKCα levels, with the consequent reduction of the intracellular cAMP levels and PKA activity. Most interestingly, it was observed that hyperplastic cholangiocytes isolated from BDL rats are able to synthesize and secrete serotonin.[58] If, in vitro or in vivo, cholangiocyte-secreted serotonin is neutralized, cholangiocyte proliferation in response to cholestasis further increases[58]. This study, therefore, proposes an additional novel working model (as shown in Figure 1): in the course of cholestasis, cholangiocytes acquire a neuroendocrine phenotype that allow them to synthesize some neuroendocrine hormones, like serotonin. Once secreted, these hormones aim to counterbalance the excessive proliferative response of the biliary epithelium. It is thus possible to postulate the existence of an autocrine/paracrine loop of peptides and neuroendocrine hormones that modulate with a negative (or an eventually positive) feed-back the response of cholangiocytes to liver injury[58]. The investigation of the presence of other peptides and hormones that are likely to participate with serotonin in this autocrine/paracrine loop might thus be needed to understand certain still obscure clinical aspects of cholangiopathies.
Recent evidence demonstrated that a “stimulatory” autocrine/paracrine loop should also exist. It has been found that cholangiocytes are the target of the growth hormone (GH)-insulin like growth factor (IGF)-1 axis[59]: GH induces IGF-1 expression and release in isolated cholangiocytes, with the consequent stimulation of cell growth by IGF-1[59].
| Peptide | Receptor | Effect on cholangiocyte biology | Reference |
| Acetylcholine | M3 | Potentiates the effect of secretin. Required for cholangiocyte response to BDL: sustains cholangiocyte proliferation and prevents apoptosis | 43, 60, 61, 64, 65 |
| Epinephrine/ Norephinephrine | α1 | Potentiates the effect of secretin | 67 |
| Epinephrine/ Norephinephrine | β1-β2 | Required for cholangiocyte response to BDL: sustains cholangiocyte proliferation and prevents apoptosis | 70 |
| Dopamine | D2 | Inhibits secretin-induced ductal secretion | 73 |
| NGF | TrkA | Secreted by hyperplastic, cholestatic cholangiocytes; sustains the rpoliferative response to BDL | 74 |
A number of studies showed that cholinergic nerves regulate bile secretion[60,61]. In bile-fistula dogs with interrupted enterohepatic circulation, distal stimulation of the vagus nerve increases bile bicarbonate secretion, whereas vagotomy decreases basal bile flow and bicarbonate output[60,61].
In early studies[62] it seemed that cholangiocytes might express both the M1 and the M3 acetylcholine (ACh) receptor subtypes and that the cholinergic agonist, carbachol, stimulates bile flow of the isolated perfused rat liver. These observations were not confirmed by successive investigations. In 1996, Nathanson et al demonstrated that, in accordance with the concept that cholangiocytes, but not hepatocytes, express ACh receptors, ACh did not affect the functions of hepatocytes, but elicited Ca2+ increase and oscillation in IBDUs, due to both an influx of extracellular Ca2+ and the mobilization of thapsigargin-sensitive Ca2+ stores[63]. A year later, Alvaro et al showed that cholangiocytes express, at the basolateral domain, M3 (but not M1 and M2) Ach receptors.[43] In that study it was shown that ACh has no effect on the basal activity of the Cl-/HCO3- exchanger, but it significantly potentiates the stimulatory effect of secretin on this anion exchanger[43]. Presumably, the significance of ACh-regulation of bicarbonate secretion by bile ducts is to be researched in the need of bicarbonate-enriched secretion in the small intestine during the digestive phase, when, indeed, the parasympathetic system activity is high. By selectively interacting with M3 receptor subtypes, ACh induces a Ca2+-calcineurin mediated potentiation of the secretin-induced adenylyl cyclase activity, which leads to activation of the Cl-/HCO3- exchanger with bicarbonate secretion into bile[43]. Furthermore, recent studies have shown that ACh sustains cholangiocyte proliferation, since interruption of the cholinergic innervation by vagotomy induces a marked decrease in total bile duct mass caused by both impaired cholangiocyte proliferative capacity and intracellular cAMP levels, and enhanced cell death by apoptosis[64,65]. These studies also show that maintenance of intracellular cAMP levels (by chronic administration of forskolin) prevents the effects of vagotomy on cholangiocyte apoptosis, proliferation and secretion[65].
There is growing information regarding the role of adrenergic and dopaminergic innervation in the regulation of cholangiocyte proliferation and secretion. An intact sympathetic innervation is required for hepatocyte and cholangiocyte proliferation following partial hepa-tectomy[66]. Recent studies demonstrated that: (1) cholangiocytes from BDL rats express alpha-1, alpha-2, beta-1 and beta-2 adrenergic receptors; and (2) alpha-1 (but not beta-1) adrenergic receptor agonists increase secretin-stimulated ductal secretion[67]. Similar to what is shown in the gut, adrenergic innervation may play a role in counterbalancing the stimulatory effects of cholinergic nerves[65] on ductal bile secretion in chronic cholestatic liver diseases.
In support of the concept that adrenergic innervation plays an important role in the regulation of cholangiocyte functions, administration of a single intraportal injection of 6-hydroxidopamine, which induces degeneration of dopaminergic terminal fibers[68,69], blunts the cholangiocyte functional and proliferative response to cholestasis and induces cell death by apoptosis[70]. Chronic administration of clenbuterol (a beta-2 adrenergic agonist)[71] and dobutamine (a beta-1 adrenergic agonist)[72] prevents the decrease in cAMP levels and secretion induced by 6-OHDA, maintains cholangiocyte proliferation and decreases cholangiocyte apoptosis due to 6-OHDA[70]. Furthermore, it has been shown that cholangiocytes express the D2 (but not the D1 and D3) dopaminergic receptors and that the D2 dopaminergic agonist, quinelorane inhibits secretin-induced ductal secretion in BDL rats through activation of the Ca2+-dependent PKC gamma (but not PKC alpha, beta I and II) and inhibition of secretin-stimulated cAMP levels and PKA activity[73].
The relationship between the biliary epithelium and nerves appears to be more than what was thought at the beginning. Cholangiocytes express the receptor for neurotrophin Nerve Growth Factor (NGF), the activation of which strongly promotes biliary cell growth[74]. Moreover, it has been found that cholangiocytes themselves can produce and secrete NGF and that when this neurotrophin is immunoneutralized the growth of the biliary tree in the BDL rat is strongly diminished[74].
Altogether, these data suggest that the autonomic innervation plays a substantial role in the regulation of cholangiocyte biology, thus enlightening the need of studying whether the liver denervation after transplantation might affect the functions of the grafted biliary tree. Of major interest would also be to investigate whether other endogenous factors, instead of nerves, can support cholangiocyte functions in the denervated organ. In this view, some evidence suggest that bile acids could be important. It has been found that administration of taurocholic[64] or ursodeoxycholic acid[75] counteracts the loss of bile ducts induced by cholinergic denervation in the BDL rat. Similarly, taurocholic acid administration also prevents the loss of bile ducts induced by adrenergic denervation[76].
Cholangiocarcinoma, the primary cancer that originates from the epithelial cells lining the bile ducts, is a devastating malignancy characterized by poor prognosis and high mortality[6,77]. The only curative treatment, even if only possible at an early stage of this neoplasm, is surgical. However, patients often present this tumor at an advanced stage, when a curative surgery is unlikely. In fact, at the time of diagnosis, more than two-thirds of patients affected by biliary tract cancer present as an unresectable disease[78] and patients with an operable tumor have a high rate of recurrence. Overall survival rate, including resected patients is quite poor, with less than 5% of patients surviving 5 years, a rate which has remained unchanged over the past 30 years[77]. Chemotherapy and radiation therapy have been used in an attempt to control this disease and improve survival and quality of life of patients with unresectable, recurrent and metastatic cholangiocarcinoma, but these therapies have not shown to be effective in prolonging long-term survival. For these reasons there is a compelling need to discover novel molecules and agents to target the cholangiocarcinoma cells and to regulate their destructive growth[79]. There is growing information regarding the role of nerves and neuropeptides as modulators of cholangiocyte function and metabolism[14]. Moreover, several reports suggest that nervous stimuli are involved in the regulation of growth of biliary malignancies[16,17,80].
| Hormone | Receptor | Effect on cholangiocarcinoma cell biology | Reference |
| Estrogens | ER | Stimulated SK-ChA-1 human cholangiocarcinoma cell growth | 81 |
| Tamoxifen induced dose-dependent growth inhibition of OZ and SK-ChA-1 human cells in vitro; reduced growth of a SK-ChA-1 tumor cell xenografts implanted in athymic nude mice | 81 | ||
| Tamoxifen stimulated SK-ChA-1 human cholangiocarcinoma apoptotic cell death | 82 | ||
| Tamoxifen induced human cholangi-ocarcinoma cell apoptosis in vitro and inhibited tumor xenograft growth after pretreatment with IFN-gamma | 83, 84 | ||
| Gastrin | CCK-B/gastrin | Inhibited Mz-ChA-1, HuH-28, and TFK-1 human cell lines proliferation and induced Mz-ChA-1 cell apoptosis | 90 |
| CCK | CCK | Reduced the growth of SLU-132 human tumor xenografts implanted in nude mice; stimulated the release of carcinoembryonic antigen (CEA) by SLU-132 cells | 93 |
| Hormone | Receptor | Effect on cholangiocarcinoma cell biology | Reference |
| Somatostatin, analogues (octreotide, lanreotide) | SSTR2 | Inhibited human cholangiocarcinoma cell proliferation in vitro and reduced human cholangiocarcinoma cell growth implanted in athymic mice | 96 |
| Inhibited RBE, NEC, QBC939, and SSP-25 cell proliferation in vitro through cell cycle arrest and QBC939 xenografts growth | 95 | ||
| GABA | GABAA,-B,-C | Inhibited human Mz-ChA-1, HuH-28 and TFK-1 cell proliferation in vitro; reduced malignant cholangiocyte migration. Reduced Mz-ChA-1 xenograft tumor growth implanted in athymic mice | 98 |
| NPY | NPY-Y5 | Inhibited Mz-ChA-1 cell proliferation in vitro | 99 |
The involvement of neuroendocrine system in regulating cholangiocarcinoma cell proliferation has been suggested by several studies. Tamoxifen, an estrogen antagonist, cause a dose-dependent decrease of proliferation of two human cholangiocarcinoma cell lines in vitro[81]. On the other hand, 17-β estradiol stimulate human cholangiocarcinoma cell growth in vitro[81]. In addition, tamoxifen induced a growth reduction of a cholangiocarcinoma tumor implanted in athymic nude mice[81]. Tamoxifen exerts its inhibitory effect in human cholangiocarcinoma cells by stimulating apoptotic cell death and this is likely mediated through the Fas/APO-1 (CD95) signaling pathway via a calmodulin-dependent mechanism[82].
A further study showed that tamoxifen exposure to human cholangiocarcinoma after pre-treatment with IFN-gamma allows for induction of apoptosis in vitro and significant inhibition of tumor growth[83]. Thus, the combination of IFN-gamma and estrogen antagonists, including tamoxifen, could be useful to have a sustained and valid inhibitory effect on cholangiocarcinoma cell growth[83,84].
Gastrointestinal polypeptide hormones regulate the growth of various normal gastrointestinal tissues as well as certain visceral cancers. Gastrin, which belongs to the superfamily of cholecystokinin receptors (CCK-A, CCK-B/gastrin receptors), is a trophic factor within the normal gastrointestinal tract and is also a mitogen for a number of gastrointestinal and non-gastrointestinal tumors such as gastric, colonic and pancreatic[85-88]. In contrast, CCK-B/gastrin receptor signaling in the human pancreatic cell lines MiaPaca-2 and Panc-1 leads to inhibition of cell growth[89]. It has been shown that malignant cholangiocytes express gastrin receptors[90] and that gastrin, interacting with CCK-B/gastrin receptors, inhibited the proliferation of Mz-ChA-1, HuH-28 and TFK-1 cholangiocarcinoma cell lines, also inducing apoptosis in Mz-ChA-1 cells by activation of the Ca2+-dependent PKC-α signaling[90]. The blockage of CCK-B receptors did not totally reverse the inhibitory effect of gastrin on Mz-ChA-1 cell growth. This finding could be explained by the fact the gastrin may exert its effect not only through CCK-B/gastrin receptors, but also through other receptor types, as suggested by studies in other carcinomas cell lines (e.g. colonic cancer cells)[91]. This partial inhibitory effect by CCK-B/gastrin receptor inhibitors might also be due to altered processing of the CCK/B receptor by malignant transformed cells, as previously described[92]. A previous study by Hudd et al showed that human cholangiocarcinoma cells expressed CCK receptors and chronic treatment with CCK octapeptide reduced the growth of human cholangiocarcinoma tumors implanted in nude mice[93].
Somatostatin receptors (SS), most commonly the SS receptor type 2 (SSTR2), have been described in several neuroendocrine and epithelial malignancies[94], such as in malignant cholangiocytes[95,96]. Studies demonstrated that somatostatin and its analogues inhibit in vitro cholangiocarcinoma cell proliferation. Indeed, chronic administration of lanreotide, a long-acting SS analogue, reduces the growth of human cholangiocarcinoma cells when implanted in athymic mice[95,96] and the inhibitory effect of somatostatin in cholangiocarcinoma cell growth was accompanied by no changes in cellular cyclic adenosine monophosphate (cAMP) or calcium intracellular levels. Furthermore, Zhao et al showed that octreotide inhibits cholangiocarcinoma cells growth through G0/G1 cell cycle arrest rather than through the process of apoptosis. These effects were partially mediated by enhancing the expression of p27kip1, and by decreasing the amounts of cyclin E-CDK2 complex[95]. Taken together, these findings suggested possible use of SS analogues in the diagnosis or therapy of cholangiocarcinoma. However, a subsequent phase II study showed absence of therapeutic efficacy of the somatostatin analogue lanreotide in the treatment of advanced primary hepatic cholangiocellular cancer and gallbladder adenocarcinoma, despite in vivo somatostatin-receptor expression[97].
Liver represents the most important site of GABA synthesis and metabolism outside the central nervous system. Recently, our group demonstrated that cholangio-carcinoma cells express GABAA,-B,-C recep-tors and respond to GABA stimulation with growth inhibition. GABA inhibitory effect on malignant cholangiocyte proliferation was evident in vitro and also in vivo, by reducing the growth of cholangiocarcinoma tumors injected subcutaneously in nude mice. Moreover, GABA has been shown to be able to inhibit malignant cholangiocyte migration, a peculiar characteristic of the cholangiocarcinoma cells[98].
Another neurotransmitter available in the liver parenchyma and biliary tract is the neuropeptide Y (NPY)[99]. Recent preliminary data from our group showed that NPY inhibits cholangiocarcinoma growth by interaction with a G-protein coupled receptor by Ca2+ dependent modulation of Src/ERK1/2 phosphorylation[99].
| Peptide | Receptor | Effect on cholangiocarcinomacell biology | Reference |
| Epinephrine/ Norephinephrine | α2 | UK 14,304 inhibited human Mz-ChA-1 and TFK-1 cell proliferation in vitro | [80] |
| Acetylcholine | M1 | Carbachol produced an increase of IP3 and Ca2+ intracellular levels in Mz-ChA-1 cells | [62] |
Autonomic nervous system regulates the growth of several tumors[100-102]. For example, alpha-blockers, terazosin and doxazosin, suppress prostate growth by inducing apoptosis among the epithelial cells in the benign and malignant prostate[103]. Also, phenylephrine reduced HepG2 cell growth through alpha1B-adrenergic receptors activation[104]. Moreover, activation of a β-2 adrenergic receptor/Gsα fusion protein leads to the inhibition of cAMP-sensitive S49 lymphoma and carcinoma carB cells proliferation
in vitro[105].
A specific role of sympathetic nervous system in the regulation of cholangiocarcinoma growth has been described by Kanno et al, that showed that cholangiocarcinoma cell lines Mz-ChA-1 and TFK-1 express the α2A-, α2B-, α2C- adrenergic receptor subtypes[80]. Furthermore, stimulation of malignant cholangiocytes by UK14, 304, an α2-adrenoreceptor agonist, causes up-regulation of cAMP, which inhibits EGF-induced MAPK activity through an acute increase of Raf-1 and sustained activation of B-Raf[80], thus reducing tumor cell proliferation. Since inhibition of cholangiocarcinoma cell growth through activation of α2-adrenergic receptor occurred downstream to Ras, this study suggested that adrenergic stimulation or other stimulants of cAMP may overcome the Ras mutations present in malignant cholangiocytes and offer a new therapeutic approach in patients with cholangiocarcinoma[80].
Other studies showed that Mz-ChA-1 cells express muscarinic acetylcholine (ACh) receptors[62]. In support of the hypothesis that such receptors modulate cho-langiocarcinoma cell growth, stimulation of malignant biliary cells with carbachol, a muscarinic acetylcholine receptor agonist, triggers IP3 formation and a subsequent increase of intracellular Ca2+ levels[62]. Even if several studies show IP3 and Ca2+ levels play an important role in the inhibition of cholangiocarcinoma cell growth after stimulation with several molecules[13,90,98]. the specific action of parasympathetic nervous system in modulating cholangiocarcinoma cell growth is still unknown.
However, recent data showed that muscarinic receptors are directly activated by other molecules. In fact, recent studies showed that bile acids, interacting with M3 Ach receptors, transactivate EGFR and stimulate colon cancer cell proliferation by inducing p90RSK phosphorylation via a calcium-, MEK- and MAPK-dependent pathways[106].
Recent findings suggest that cholangiocarcinoma and other liver tumors could arise from activated hepatic progenitor cells[107-109]. Several evidences indicate that hepatic progenitor cell activation in diseased liver is regulated by neural and neuroendocrine factors such as vagal innervation[110,111], suggesting an important involvement of autonomic nervous system in developing biliary carcinogenesis.
The amount of evidences indicating the substantial role of neuroendeocrine hormones, neuropeptides and neurotransmitters in modulating malignant and non malignant cholangiocyte biology is rapidly increasing. There is still much to understand on how actually these peptides create a network that affects the response of biliary cells to liver injury. On the other hand the other frontier is no doubt represented by the identification of the most proper way of modulating such a network of peptides. Will we be able to interfere with it in order to divert or relent the progression of cholangiopathies and cholangiocarcinoma
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
| 1. | Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Desmet VJ. Vanishing bile duct disorders. Prog Liver Dis. 1992;10:89-121. [PubMed] |
| 4. | Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | 2001 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1991-2000. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation; Richmond, VA: United Network for Organ Sharing; Ann Arbor, MI: University Renal Research and Education Association. |
| 6. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 344] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Alpini G, Kanno N, Phinizy JL, Glaser S, Francis H, Taffetani S, LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G973-G982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Shetzline MA, Liddle RA. Gastrointestinal hormones and neurotransmitters. Philadelphia, U.S. : Saunders 2002; 3-20. |
| 15. | Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019-1025. [PubMed] |
| 16. | Chan C, Medina-Franco H, Bell W, Lazenby A, Vickers S. Carcinoid tumor of the hepatic duct presenting as a Klatskin tumor in an adolescent and review of world literature. Hepatogastroenterology. 2000;47:519-521. [PubMed] |
| 17. | Hsu W, Deziel DJ, Gould VE, Warren WH, Gooch GT, Staren ED. Neuroendocrine differentiation and prognosis of extrahepatic biliary tract carcinomas. Surgery. 1991;110:604-610; discussion 610-611. [PubMed] |
| 18. | Bioulac-Sage P, Lafon ME, Saric J, Balabaud C. Nerves and perisinusoidal cells in human liver. J Hepatol. 1990;10:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Oda M, Han JY, Nakamura M. Endothelial cell dysfunction in microvasculature: relevance to disease processes. Clin Hemorheol Microcirc. 2000;23:199-211. [PubMed] |
| 20. | Püschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:854-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Schneider DA, Sayegh AI. Gastrointestinal neuroendocrinology. Vet Clin North Am Equine Pract. 2002;18:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Le Douarin NM. On the origin of pancreatic endocrine cells. Cell. 1988;53:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Reilly FD, McCuskey PA, McCuskey RS. Intrahepatic distribution of nerves in the rat. Anat Rec. 1978;191:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Tsuneki K, Ichihara K. Electron microscope study of vertebrate liver innervation. Arch Histol Jpn. 1981;44:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Gulbenkian S, Wharton J, Hacker GW, Varndell IM, Bloom SR, Polak JM. Co-localization of neuropeptide tyrosine (NPY) and its C-terminal flanking peptide (C-PON). Peptides. 1985;6:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Lundberg JM, Terenius L, Hökfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 936] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 27. | Costa M, Furness JB. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984;13:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985;57:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 477] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Julé Y, Clerc N, Niel JP, Condamin M. [Met]- and [Leu]enkephalin-like immunoreactive cell bodies and nerve fibres in the coeliac ganglion of the cat. Neuroscience. 1986;18:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Schultzberg M, Dalsgaard CJ. Enteric origin of bombesin immunoreactive fibres in the rat coeliac-superior mesenteric ganglion. Brain Res. 1983;269:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Burt AD, Tiniakos D, MacSween RN, Griffiths MR, Wisse E, Polak JM. Localization of adrenergic and neuropeptide tyrosine-containing nerves in the mammalian liver. Hepatology. 1989;9:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Inoue N, Sakai H, Magari S, Sakanaka M. Distribution and possible origins of substance P-containing nerve fibers in the rat liver. Ann Anat. 1992;174:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Goehler LE, Sternini C, Brecha NC. Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea-pig: distribution and colocalization with substance P. Cell Tissue Res. 1988;253:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612-G625. [PubMed] |
| 35. | Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Alpini G, Ulrich CD 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol. 1994;266:G922-G928. [PubMed] |
| 38. | Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage GD, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol. 1997;272:G289-G297. [PubMed] |
| 39. | Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767-G775. [PubMed] |
| 40. | Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol. 1997;273:G1061-G1070. [PubMed] |
| 41. | Alvaro D, Mennone A, Boyer JL. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3- exchange in cholangiocytes. Am J Physiol. 1997;273:G303-G313. [PubMed] |
| 42. | Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993;91:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser SS. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Tietz PS, Alpini G, Pham LD, Larusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol. 1995;269:G110-G118. [PubMed] |
| 45. | Gong AY, Tietz PS, Muff MA, Splinter PL, Huebert RC, Strowski MZ, Chen XM, LaRusso NF. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol. 2003;284:C1205-C1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Lesage GD, Marucci L, Alvaro D, Glaser SS, Benedetti A, Marzioni M, Patel T, Francis H, Phinizy JL, Alpini G. Insulin inhibits secretin-induced ductal secretion by activation of PKC alpha and inhibition of PKA activity. Hepatology. 2002;36:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Caligiuri A, Glaser S, Rodgers RE, Phinizy JL, Robertson W, Papa E, Pinzani M, Alpini G. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol. 1998;275:G835-G846. [PubMed] |
| 48. | Cho WK, Boyer JL. Vasoactive intestinal polypeptide is a potent regulator of bile secretion from rat cholangiocytes. Gastroenterology. 1999;117:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Cho WK, Mennone A, Rydberg SA, Boyer JL. VIP is a potent stimulus of bicarbonate and fluid secretion in bile ducts. Hepatology. 1995;22:A752. |
| 50. | Cho WK, Mennone A, Rydberg SA, Boyer JL. Bombesin stimulates bicarbonate secretion from rat cholangiocytes: implications for neural regulation of bile secretion. Gastroenterology. 1997;113:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdury U, Papa E. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Alvaro D, Alpini G, Onori P, Franchitto A, Glaser S, Le Sage G, Gigliozzi A, Vetuschi A, Morini S, Attili AF. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology. 2002;123:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Alvaro D, Invernizzi P, Onori P, Franchitto A, De Santis A, Crosignani A, Sferra R, Ginanni-Corradini S, Mancino MG, Maggioni M. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol. 2004;41:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Jones EA, Bergasa NV. The pathogenesis and treatment of pruritus and fatigue in patients with PBC. Eur J Gastroenterol Hepatol. 1999;11:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Swain MG, Maric M. Improvement in cholestasis-associated fatigue with a serotonin receptor agonist using a novel rat model of fatigue assessment. Hepatology. 1997;25:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Kaminski DL, Dorighi J, Jellinek M. Effect of electrical vagal stimulation on canine hepatic bile flow. Am J Physiol. 1974;227:487-493. [PubMed] |
| 61. | Tanturi C, Ivy A. On the existence of secretory nerves in the vagi for and the reflex excitation and inhibition of bile secretion. Am J Physiol. 1938;121:270-283. |
| 62. | Elsing C, Hübner C, Fitscher BA, Kassner A, Stremmel W. Muscarinic acetylcholine receptor stimulation of biliary epithelial cells and its effect on bile secretion in the isolated perfused liver [corrected]. Hepatology. 1997;25:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol. 1996;271:G86-G96. [PubMed] |
| 64. | Marzioni M, LeSage GD, Glaser S, Patel T, Marienfeld C, Ueno Y, Francis H, Alvaro D, Tadlock L, Benedetti A. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct-ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837-G852. [PubMed] |
| 65. | LeSagE G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Iwai M, Shimazu T. Alteration in sympathetic nerve activity during liver regeneration in rats after partial hepatectomy. J Auton Nerv Syst. 1992;41:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | LeSage GD, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Elliott KJ, Jones JM, Sacaan AI, Lloyd GK, Corey-Naeve J. 6-hydroxydopamine lesion of rat nigrostriatal dopaminergic neurons differentially affects nicotinic acetylcholine receptor subunit mRNA expression. J Mol Neurosci. 1998;10:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Lindfeldt J, Zoucas E, Ekelund M, Kullendorff CM, Holmin T. The effect of intraportal six-hydroxy-dopamine on hemorrhage after standardized liver trauma in rats. J Trauma. 1987;27:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, Marzioni M, Mancino MG, Phinizy JL. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol. 2006;290:G813-G826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Hinkle RT, Hodge KM, Cody DB, Sheldon RJ, Kobilka BK, Isfort RJ. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve. 2002;25:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Barnes E, Dutka DP, Khan M, Camici PG, Hall RJ. Effect of repeated episodes of reversible myocardial ischemia on myocardial blood flow and function in humans. Am J Physiol Heart Circ Physiol. 2002;282:H1603-H1608. [PubMed] |
| 73. | Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683-G694. [PubMed] |
| 74. | Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, Francis H, Mancino MG, Ueno Y, Barbaro B. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Taffetani S, Alvaro D, Phinizy JL, Baumann B, Venter J. Taurocholate feeding prevents the functional damage of intrahepatic bile ducts induced by adrenergic denervation in a PI3K dependent manner. Hepatology. 2003;38:A28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 77. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 899] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 78. | Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16 Suppl 2:ii93-ii96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Kanno N, Lesage G, Phinizy JL, Glaser S, Francis H, Alpini G. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Sampson LK, Vickers SM, Ying W, Phillips JO. Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res. 1997;57:1743-1749. [PubMed] |
| 82. | Pan G, Vickers SM, Pickens A, Phillips JO, Ying W, Thompson JA, Siegal GP, McDonald JM. Apoptosis and tumorigenesis in human cholangiocarcinoma cells. Involvement of Fas/APO-1 (CD95) and calmodulin. Am J Pathol. 1999;155:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Vickers SM, Jhala NC, Ahn EY, McDonald JM, Pan G, Bland KI. Tamoxifen (TMX)/Fas induced growth inhibition of human cholangiocarcinoma (HCC) by gamma interferon (IFN-gamma). Ann Surg. 2002;235:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Ahn EY, Pan G, Oh JH, Tytler EM, McDonald JM. The combination of calmodulin antagonists and interferon-gamma induces apoptosis through caspase-dependent and -independent pathways in cholangiocarcinoma cells. Am J Pathol. 2003;163:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Ishizuka J, Martinez J, Townsend CM Jr, Thompson JC. The effect of gastrin on growth of human stomach cancer cells. Ann Surg. 1992;215:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Yassin RR, Clearfield HR, Little KM. Gastrin's trophic effect in the colon: identification of a signaling pathway mediated by protein kinase C. Peptides. 1993;14:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 247] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Watson SA, Steele RJ. Gastrin antagonists in the treatment of gastric cancer. Anticancer Drugs. 1993;4:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Detjen K, Fenrich MC, Logsdon CD. Transfected cholecystokinin receptors mediate growth inhibitory effects on human pancreatic cancer cell lines. Gastroenterology. 1997;112:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Bold RJ, Alpard S, Ishizuka J, Townsend CM Jr, Thompson JC. Growth-regulatory effect of gastrin on human colon cancer cell lines is determined by protein kinase a isoform content. Regul Pept. 1994;53:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Cookson MS, Reuter VE, Linkov I, Fair WR. Glutathione S-transferase PI (GST-pi) class expression by immunohistochemistry in benign and malignant prostate tissue. J Urol. 1997;157:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Hudd C, Euhus DM, LaRegina MC, Herbold DR, Palmer DC, Johnson FE. Effect of cholecystokinin on human cholangiocarcinoma xenografted into nude mice. Cancer Res. 1985;45:1372-1377. [PubMed] |
| 94. | Eden PA, Taylor JE. Somatostatin receptor subtype gene expression in human and rodent tumors. Life Sci. 1993;53:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Zhao B, Zhao H, Zhao N, Zhu XG. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J Hepatobiliary Pancreat Surg. 2002;9:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Tan CK, Podila PV, Taylor JE, Nagorney DM, Wiseman GA, Gores GJ, LaRusso NF. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Fiebiger WC, Scheithauer W, Traub T, Kurtaran A, Gedlicka C, Kornek GV, Virgolini I, Raderer M. Absence of therapeutic efficacy of the somatostatin analogue lanreotide in advanced primary hepatic cholangiocellular cancer and adenocarcinoma of the gallbladder despite in vivo somatostatin-receptor expression. Scand J Gastroenterol. 2002;37:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437-11446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Fava G, Glaser S, Francis H, Phinizy J, Venter J, Reichenbach R, Taffetani S, Marzioni M, Marucci L, Benedetti A. Neuropeptide Y (NPY) inhibits cholangiocarcinoma growth by interaction with a G-protein coupled receptor by a Ca2 dependent modulation of Src/ERK1/2 phosphorylation. Gastroenterology. 2004;126 Suppl 2. |
| 100. | Cardani R, Zavanella T. Decreased density of beta1-adrenergic receptors in preneoplastic and neoplastic liver lesions of F344 rats. Histol Histopathol. 2005;20:843-850. [PubMed] |
| 101. | Fernando MA, Heaney AP. Alpha1-adrenergic receptor antagonists: novel therapy for pituitary adenomas. Mol Endocrinol. 2005;19:3085-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Roumeguère T. [Novel therapeutics strategies in benign hypertrophy of prostate management]. Rev Med Brux. 2005;26:S407-S411. [PubMed] |
| 103. | Kyprianou N, Chon J, Benning CM. Effects of alpha(1)-adrenoceptor (alpha(1)-AR) antagonists on cell proliferation and apoptosis in the prostate: therapeutic implications in prostatic disease. Prostate Suppl. 2000;9:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | Auer KL, Spector MS, Tombes RM, Seth P, Fisher PB, Gao B, Dent P, Kunos G. Alpha-adrenergic inhibition of proliferation in HepG2 cells stably transfected with the alpha1B-adrenergic receptor through a p42MAPkinase/p21Cip1/WAF1-dependent pathway. FEBS Lett. 1998;436:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Bertin B, Jockers R, Strosberg AD, Marullo S. Activation of a beta 2-adrenergic receptor/Gs alpha fusion protein elicits a desensitization-resistant cAMP signal capable of inhibiting proliferation of two cancer cell lines. Receptors Channels. 1997;5:41-51. [PubMed] |
| 106. | Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Lee JH, Rim HJ, Sell S. Heterogeneity of the "oval-cell" response in the hamster liver during cholangiocarcinogenesis following Clonorchis sinensis infection and dimethylnitrosamine treatment. J Hepatol. 1997;26:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Robrechts C, De Vos R, Van den Heuvel M, Van Cutsem E, Van Damme B, Desmet V, Roskams T. Primary liver tumour of intermediate (hepatocyte-bile duct cell) phenotype: a progenitor cell tumour. Liver. 1998;18:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F, Bannasch P. Oval cell lines OC/CDE 6 and OC/CDE 22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest. 1994;71:700-709. [PubMed] |
| 110. | Cassiman D, Libbrecht L, Sinelli N, Desmet V, Denef C, Roskams T. The vagal nerve stimulates activation of the hepatic progenitor cell compartment via muscarinic acetylcholine receptor type 3. Am J Pathol. 2002;161:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |