INTRODUCTION
The prevalence of constipation in adults ranges from 2% to 27% in North America, particularly after the age of 65 and with a female predominance[1]. There are two major types of constipation: secondary constipation (due to colorectal cancer, hypothyroidism, hyperparathyroidism, diabetes, use of opiates, antidepressants, and diuretics, stool impaction in the elderly, sexual abuse in women, Parkinson’s disease, and other organic pathology and medications) and primary functional constipation, which can be divided into slow transit constipation (colonic inertia), constipation-predominant irritable bowel syndrome, and obstructed defecation[2,3]. Approximately half of constipated patients suffer from obstructed defecation[4]. Obstructed defecation (OD)is a broad term of the pathophysiologic condition describing the inability to evacuate contents from the rectum. This disorder is commonly known by numerous terms, including paradoxical puborectalis contraction (PPC), outlet obstruction, anismus, pelvic floor dyssynergia, nonrelaxing puborectalis syndrome, spastic pelvic floor syndrome, and dyschezia. It may result from functional, metabolic, mechanical, and anatomical derangements involving a rectoanal evacuatory mechanism. Dyssynergia, or uncoordination of the pelvic floor muscles, leads to paradoxical external anal sphincter and puborectalis contraction with no relaxation during defecation[5].
OD may result from rectoanal intussusception, pelvic organ prolapse, rectocele, sigmoidocele, enterocele, solitary rectal ulcer syndrome, PPC and descending perineum syndrome. Other rare causes include rectal hyposensitivity (blunted rectum), idiopathic megarectum, hereditary internal sphincter myopathy and nutcracker anus[4,6].
This condition is usually defined on the basis of symptoms and physiologic and radiologic studies. Symptoms include a feeling of incomplete evacuation and rectal obstruction, passage of hard stools, rectal or vaginal digitation and excessive straining in the constipated patient (stool frequency of fewer than three times per week). Careful perineal and digital rectal examination, colonic transit time study, anorectal manometry, defecography or dynamic magnetic resonance imaging of the pelvic floor usually help assess defecatory dysfunction and establish a correct diagnosis[7,8].
Fecal incontinence (FI) is a second major problem with a devastating effect on patients’ quality of life. Its prevalence ranges from 2% to 18% and increases with age especially in institutionalized and psychiatric patients and in women[9]. FI is usually attributable to multiple factors affecting the normal anatomy and physiology of anorectum. Obstetric trauma, anorectal surgery, benign colorectal conditions, cancer, inflammatory bowel and neurologic diseases, aging, drugs, food intolerance, rectal prolapse, congenital abnormalities, and radiation proctitis are all known causes of FI. The most appropriate therapy for FI may be based only on an accurate assessment of anorectal function, including a thorough history, physical examination, anorectal physiology studies (endoanal ultrasound, anorectal manometry with balloon expulsion test, electromyography, and pudendal nerve latency testing) and other imaging tests (defecography, dynamic MR proctography)[10]. The main goal of treatment is to improve continence and the patients’ quality of life.
TREATMENT STRATEGY
Obstructed defecation
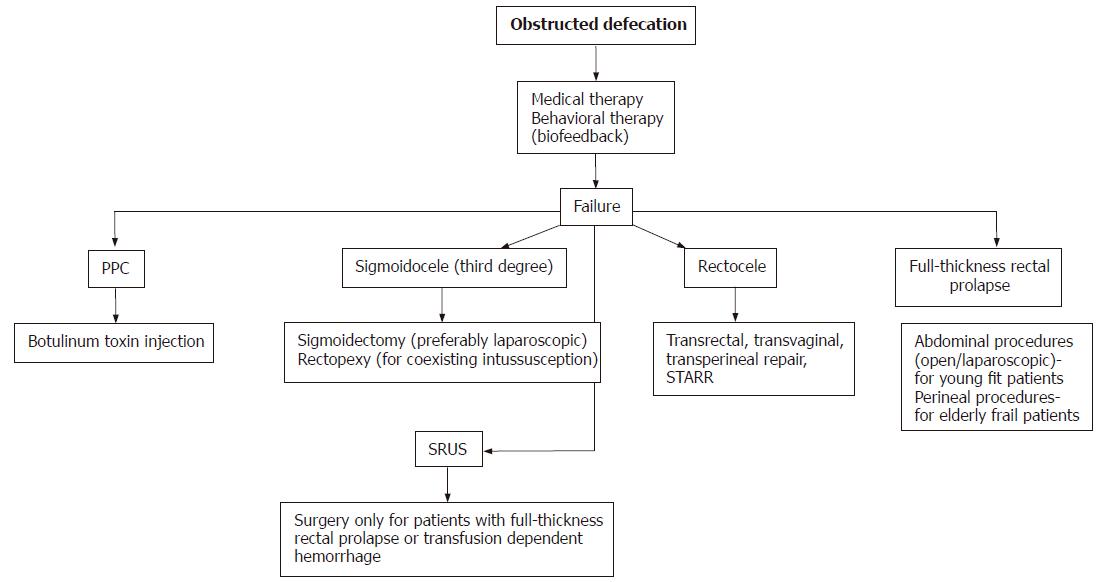
Once secondary causes for constipation, especially malignancy and slow transit constipation (colonic inertia) are excluded, OD is considered on the basis of clinical examination, normal or prolonged colonic transit time, abnormal anorectal manometry, defecography, or dynamic MRI of the pelvic floor[11] (Figure 1).
Figure 1 Treatment algorithm for obstructed defecation.
Paradoxical puborectalis syndrome
Initial therapy for patients with PPC, as for descending perineum syndrome, solitary rectal ulcer, clinically non-significant rectocele, sigmoidocele, and rectoanal intussusception, is conservative. This includes a high fiber diet of up to 20-30 g/d, adequate hydration, regular physical activity, enemas, and laxatives.
Biofeedback training may be added to the first-line therapy, which teaches patients to relax their pelvic floor muscles[12-14]. There is a paucity of controlled trials showing the true effectiveness of this behavioral treatment. Furthermore, there is no single factor that can predict a favorable outcome with this treatment. In a large retrospective study from Cleveland Clinic Florida, Gilliland et al reported a 63% success rate of EMG-based biofeedback for constipation after five or more sessions[12]. They found that age, gender, duration of symptoms, and the coexistence of rectal pain did not influence outcome. In addition, there was no relationship between mean resting or squeeze pressure, length of the high pressure zone, sensory threshold, and maximum rectal capacity and success or failure of biofeedback. Chiarioni et al showed improvement in 71% of patients with OD for a period of two years[15]. Various biofeedback techniques (intra-anal and perianal EMG monitoring, manometric anal probe biofeedback, intra-rectal balloon expulsion biofeedback, ultrasound biofeedback) have been done, but none has a superior success rate, which ranges from 30% to over 90%[16-18]. Although the success rate seems to significantly wane with time, the morbidity-free nature of biofeedback makes re-treatment an attractive option.
Despite controversy about the method of biofeedback and the number of sessions needed, it seems reasonable to use this generally safe technique as the initial treatment for OD. After failure of conservative therapy, 50%-75% of patients with PPC may benefit from botulinum toxin type A injection into the puborectalis muscle and external anal sphincter. The short-term effect (3 mo), however, may require repeated injections[19,20]. Surgical division of the puborectalis muscle is usually non-effective and currently not a recommended procedure[21]. Several other treatments exist for this disorder, including progressive anal dilatation and sacral nerve stimulation (SNS). Additional studies are needed to completely evaluate these techniques for this indication.
Solitary rectal ulcer syndrome (SRUS)
This syndrome may be associated with PPC, rectoanal intussusception, rectal prolapse, and descending perineal syndrome. Chronic straining with ischemic ulceration are components of the pathogenesis, which is still not completely clear. Biopsy from the lesion should be obtained to exclude malignancy. Treatment for this syndrome is conservative and is the same as for PPC, including high fiber and fluid intake, laxatives, suppositories, and biofeedback. Surgery is generally reserved for patients with full thickness rectal prolapse or intractable hemorrhage and should be tailored to the individual patient’s operative risk, willingness, and continence and constipation status[22,23]. Regardless of which procedure is selected, it should be safe balancing anticipated morbidity with an acceptable recurrence rate. Unfortunately, a detailed description of the different surgical techniques is beyond the scope of this article.
Descending perineum syndrome
This syndrome is abnormal perineal descent possibly as a result of consistently prolonged straining with defecation, as defined on defecography. Medical therapy with a high fiber diet, laxatives, enemas, and biofeedback is the main treatment for these patients; no viable surgical option exists.
Rectocele
Treatment of rectocele is usually indicated when a herniation of the anterior rectal wall is greater than 3 cm with significant clinical symptoms or non-emptying rectocele on defecography. However, there is no correlation between the severity of symptoms, contrast material retention, the depth of a rectocele and success of the repair[24,25]. Rectocele presents on defecography in 81% of asymptomatic women[26]. The initial treatment is the same as for disorders previously outlined. Surgery may be considered when conservative therapy fails and includes transvaginal posterior colporrhaphy, and transrectal or transperineal repair. Careful patient selection is very important for a successful outcome. Two new techniques include the double stapled Trans-Anal Rectal Resection (STARR) and single stapled Trans-Anal Prolapsectomy with Perineal Levatorplasty (STAPL) for the management of OD associated with rectocele or intussusception. These methods use one or two 33-mm circular staplers (PPH-01 Ethicon-Endosurgery, Inc, Cincinnati, OH) to perform mucosal or full-thickness circumferential transanal resection of the distal rectum. In their randomized study Boccasanta et al reported significant symptomatic improvement after STARR and STAPL in 88% and 76%, respectively, at approximately 2 years[27]. These procedures are considered safe with minimal postoperative pain and high patient satisfaction[28].
Rectal intussusception (internal rectal prolapse)
Approximately half of the patients with rectal intussusception present with clinical symptoms of OD while in 29% it is seen on defecography in asymptomatic patients[29,30]. The mainstay of therapy is dietary management and biofeedback. Surgery is reserved only for patients with combined pathology, usually FI as a result of the external anal sphincter defects or pudendal neuropathy, or if the intussusception progresses to a full thickness rectal prolapse.
Sigmoidocele and enterocele
Deep herniation of the rectovaginal peritoneal cavity containing sigmoid colon or small bowel is usually associated with other manifestations of OD, rectocele, or pelvic organ prolapse. Sigmoidocele is confirmed by defecography. Jorge et al from the Cleveland Clinic Florida suggested a sigmoidocele classification based on the degree of descent of the lowest portion of the sigmoid colon[30]. First degree sigmoidocele is located below the sacral promontory but above the pubococcygeal line, second degree is below the pubococcygeal line and above the ischiococcygeal line, and third degree is below the ischiococcygeal line. Staging of sigmoidocele is useful in determining both clinical significance and optimal treatment.
Indications for surgery are symptomatic patients with a third-degree sigmoidocele (below ischiococcygeal line) or during other pelvic surgery, using abdominal or vaginal approaches (hysterectomy, rectal prolapse, rectocele repair). Surgery includes resection of the sigmoid colon (preferably laparoscopic sigmoidectomy), and rectopexy with obliteration of the Douglas pouch; some patients may benefit from dietary and biofeedback therapy. Jorge and colleagues reported symptomatic improvement in 100% of patients who underwent surgery, but in only 33% of patients who have been conservatively treated at a mean follow-up of 23 mo[30].
Fecal incontinence
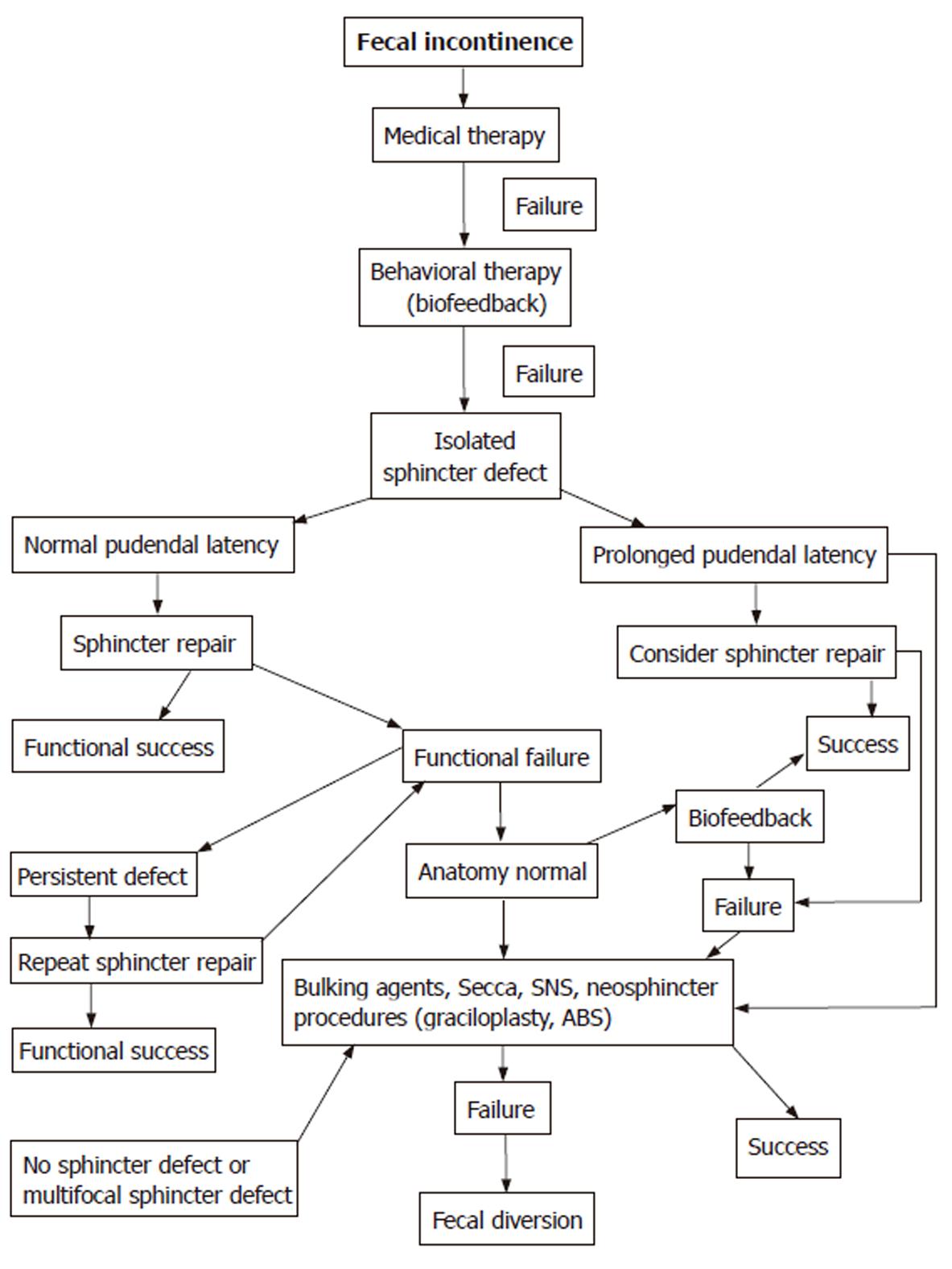
Fecal incontinence is the result of numerous disorders affecting the anatomy and physiology of the anorectum. Many appropriate medical, behavioral, and surgical treatments exist to correct the underlying pathology and restore continence. Irritable bowel syndrome, inflammatory bowel disease, scleroderma (internal anal sphincter fibrosis), neurological (spinal cord injuries) and psychological problems (dementia, depression, anxiety), diabetes mellitus (autonomic neuropathy), skeletal muscle diseases (myopathy, myasthenia gravis), diarrhea, constipation, and fecal impaction should all be appropriately treated. However, when underlying pathology cannot be identified, the primary goal of the therapy is to relieve symptoms and improve patients’ quality of life (Figure 2).
Figure 2 Treatment algorithm for fecal incontinence.
Initial therapy consists of dietary modifications to form stool, control diarrhea or constipation, and treat fecal impaction with overflow incontinence. This treatment includes perianal hygienic measures with barrier cream to prevent skin irritation and dermatitis, increased fiber and fluid uptake, avoidance of dairy products and caffeine, using stool softeners, osmotic laxatives (magnesium salts), and non-absorbable sugars (lactulose). Antidiarrheal agents (loperamide, diphenoxylate/atropine) increase fluid absorbtion, colonic transit time, and anal resting sphincter pressure[31]. Low dose amitriptyline (tricyclic antidepressant) may improve continence due to its anticholinergic and serotoninergic activities[32].
Biofeedback is another first-line therapy for FI. This method of operant conditioning, using visual verbal or auditory signals, improves rectal sensation, rectoanal coordination, and trains external anal sphincter contractility. Success rates from various series range from 40% to 85%, most probably predicted by patient motivation rather than the duration of FI, manometric or endoanal ultrasound findings, or the biofeedback technique[33,34].
Several other invasive nonoperative techniques exist that may benefit patients with FI. The Procon incontinence device (Incontinence Control Devices, Inc., Kingwood, TX) is a soft catheter with a photosensor and balloon, which may be inserted into the rectum to send a signal warning of coming stool. This device has shown an improvement in the continence of select patients[35]. Submucosal injections of a wide variety of bulking agents have been used to augment the internal anal sphincter (silicon, silicone-based agent [Bioplastique®], carbon-coated beads [AcystTM procedure], carbon-coated zirconium oxide beads [Durasphere®], autologous fat, glutaraldehyde cross-linked collagen [GAX], polytetrafluoroethylene [Polytef]) have shown symptomatic improvement in anal resting pressure and incontinence. These office-based procedures are simple, relatively safe, and seemingly efficacious[36].
The Secca® procedure (Curon Medical, Inc., Sunnyvale, CA) uses temperature-controlled radiofrequency energy in the anal canal and distal rectum to create scarring of the internal anal sphincter and tissue fibrosis. In a prospective multicenter study, Efron et al reported improvement in FI and quality of life with resolution of symptoms in 60% of patients[37,38]. Takahashi et al reported similar findings at a two-year follow-up[39].
Surgery
Failure of conservative therapy in patients with external sphincter defects but without neurologic injury (intact pudendal nerves) are suited to surgical repair. Anterior defects from obstetric damage is the most common cause of FI in these cases. The surgical procedure is usually anterior overlapping sphincteroplasty, including preservation of all fibrotic scar and overlapping of both sides of the external anal sphincter with non-absorbable sutures[40]. Although approximately 80% of patients benefit from this operation in the short-term period, the rate of incontinence increases after 5-10 years by up to 85%[41-47].
Patients with severe incontinence with significant neurologic injury, multifocal external anal sphincter damage that cannot be primarily restored, or after functional failure of an anatomical successful sphincteroplasty, may be considered for reconstruction with neosphincter procedures using striated muscles (gracilis, gluteus maximus, sartorius, adductor longus). The gracilis muscle transposition is the most commonly used technique. To prevent muscle fatigue and maintain constant contraction, this procedure involves the implantation of an electric pulse stimulator. The dynamic graciloplasty (stimulated gracilis muscle transposition) has yielded significant improvement in FI in 55% to 78% of patients [48-51]. This procedure is complex, associated with substantial morbidity, mostly infective complications, and no longer available in the United States.
The artificial bowel sphincter is an inflatable silicone cuff connected to a pump and a pressure-regulating balloon. The cuff is placed around the anal canal to maintain the basal pressure. The pump is then implanted in the scrotum or labia major. Deflation of the cuff with a shifting of fluid to the balloon, which is located behind the pubis, is controlled by the patient. One multicenter trial showed that the device was functional in 67% of patients during the first year after surgery. Despite a high rate of complications such as cuff erosion, infection in 25%, device failure and removal in 37%, and re-operations in 46%, the overall outcome is successful in more than 50% of patients[52].
Another innovative treatment of FI is sacral nerve stimulation (SNS). It stimulates both somatic and autonomic supply of the pelvic organs and anorectal region through the sacral and pudendal nerves with the neuromodulation of the sensory, motor, and autonomic innervation. However, the exact mechanism of action is unclear. Temporary electrodes are percutaneously implemented for 2-3 wk to stimulate the sacral nerve roots. If patients demonstrate significant improvement in incontinence, the leads are connected to a permanent stimulator in the subcutaneous tissue. Published results of this procedure are encouraging with marked improvement in continence in up to 100% and restored complete continence in 41%-75%[53-55]. Matzel et al published the results of a multicenter, prospective, nonrandomized trial in 37 patients with FI and showed functional improvement (reduction in incontinence episodes per week) in more than 80% of patients[54]. The SNS procedure may also be reliable in some patients with constipation.
Colostomy or ileostomy should be considered in patients with severe FI who have failed multiple procedures, are immobilized, or are considered high-risk. This procedure may be temporary to protect complex reconstructions and improve quality of life until complete distal healing, or permanent; many stomas are amenable to laparoscopic construction[56-58].
In conclusion, the management of OD is primarily medical and behavioral; biofeedback therapy is the preferred method of treatment. Except botulinum toxin injection for PPC, surgery is usually reserved for severe symptomatic patients, who have repairable anatomic defects or combined pathology. The treatment of FI should be based on the appropriate diagnosis, anorectal anatomic and physiologic investigation, and guided by the severity of incontinence. It is important to balance the anticipated improvement in the quality of life with a minimal acceptably low surgical morbidity. The first line of therapy, conservative treatment with biofeedback, is effective in the majority of patients. Well-defined external anal sphincter defects may be best repaired with sphincteroplasty, whereas radiofrequency, bulking agents, and SNS should be offered for suitable patients with more severe FI who have failed noninvasive therapy. Complex procedures such as gracilis muscle transposition and artificial bowel sphincter implantation are reserved for continued profound incontinence after failed previous surgery.