Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.302
Revised: June 18, 2005
Accepted: June 24, 2005
Published online: January 14, 2006
AIM: To investigate the roles of lymphocytes in the development of dextran sulfate sodium-induced colitis.
METHODS: Using various doses of dextran sulfate sodium (DSS), we induced colitis in wild-type B6 control and Rag-1 knockout (H-2b haplotype) mice, and evaluated the colitis in terms of symptomatic and histologic parameters, such as weight loss, survival, severity of diarrhea, shortage of colon length and histological changes. Symptomatic parameters were checked daily and histological changes were scored.
RESULTS: Although development of colitis in Rag-1 knockout mice treated with high dose (5%) of DSS was comparable to that in B6 control mice, colitis progression was much more tolerable in Rag-1 knockout mice compared to than in B6 mice treated with low dose (1.5%) DSS. Symptomatic parameters as well as histopathologic changes were improved in Rag-1 knockout mice.
CONCLUSION: These results indicate that the presence of lymphocytes contributes to colitis progression at low dose of DSS stimulation. Lymphocytes may play roles as an aggravating factor in DSS-induced colitis.
- Citation: Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, Kim JY, Oh KI. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World J Gastroenterol 2006; 12(2): 302-305
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.302
Despite many years of researches, the pathogenesis of human inflammatory bowel disease (IBD) remains poorly understood[1]. Clinical studies are limited to the patients with established disease; therefore, early events of the disease are difficult to approach. Several experimental models of colitis have been introduced to study factors that can induce chronic inflammation and investigate the evolution of colitis from the initial pathologic event to its final clinical manifestation. One of these models is based on the oral administration of dextran sulfate sodium (DSS) in the drinking water of mice, which results in an acute and chronic colitis with some morphological changes similar to human ulcerative colitis[2]. In addition, this model has been shown to respond to the anti-colitis drugs, such as sulfasalazine, olsalazine and mesalazine, that are used in human ulcerative colitis and have several immunomodulating functions[3].
The pathogenic mechanism of this colitis is attributed to its direct toxic effects to colonic epithelial cells[4], namely their relative independence from lymphocyte-mediated responses. Thus, in DSS-induced colitis, it is reported that mice lacking T cells, B cells, and NK cells can still develop colitis in response to DSS[5]. However, some studies showed the contradictory results, which suggest that lymphocytes play roles in the development of DSS-induced colitis. In acute stages of DSS-induced colitis, T cell response consists of polarized Th1 response, but in later and more chronic phase of inflammation, a mixed Th1/Th2 response occurs[6]. NKT cells, in contrast to the above CD4 T cells, were reported to play a protective role in DSS-induced colitis[7].
In this study, we investigated the role of lymphocytes in the development of DSS-induced colitis using Rag-1 knockout (KO) mice and found that the severities of the DSS-induced colitis were dramatically decreased in Rag-1 KO mice.
Eight-week-old male C57BL/6 (B6, H-2b-haplotype) and Rag-1 knockout (Rag-1 KO, H-2b-haplotype) mice were purchased from Orient (Chunduk, Korea) and Jackson laboratory (Bar Harbor, ME, USA), respectively. The mice housed in a specific pathogen-free condition at Hallym University (Chuncheon, Korea) for at least 6 weeks for adaptation to the environmental changes. All mice used in this study were 14 and 16 weeks of age. All experimental animals were cared for, maintained, and terminated in accordance with the Hallym University Guidelines.
Colitis was induced by addition of 1.5% DSS (M.W. 36,000-50,000; MP Biomedicals, Irvine, CA) to drinking water for 15 d. Animals were monitored daily for loss of body weight, diarrhea, and survival for 15 d. The severity of diarrhea was evaluated as the following scores[8]: no diarrhea = 0; mild diarrhea = 2; severe watery diarrhea = 3; mild diarrhea with blood = 4; and severe watery diarrhea with blood = 8. At 15 d or when mice had lost more than 20% of their initial body weight after DSS administration, they were sacrificed.
Colons were removed, fixed in 100 mL/L neutral formalin, embedded in paraffin, sectioned at 4-μm thickness and stained with hematoxylin and eosin (HE). Colonic epithelial damage was assigned scores as follows: 0 = normal; 1 = hyperproliferation, irregular crypts, and goblet cell loss; 2 = mild to moderate crypt loss (10-50%); 3=severe crypt loss (50-90%); 4 = complete crypt loss, surface epithelium intact; 5 = small- to medium-sized ulcer (<10 crypt widths); and 6 = large ulcer (≥10 crypt widths). Infiltration with inflammatory cells was assigned scores separately for mucosa (0 = normal, 1 = mild, 2 = modest, 3 = severe), submucosa (0 = normal, 1 = mild to modest, 2 = severe), and muscle/serosa (0 = normal, 1 = moderate to severe). Scores for epithelial damage and inflammatory cell infiltration were added, resulting in a total scoring range of 0-12[9]. All analyses were performed “blind.”
Data were expressed as mean ± SE. An unpaired Student’s t test was used to examine differences in the extent of weight loss, the survival time, and histology scores. This statistical analysis was determined using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). A P value less than 0.05 was considered statistically significant.
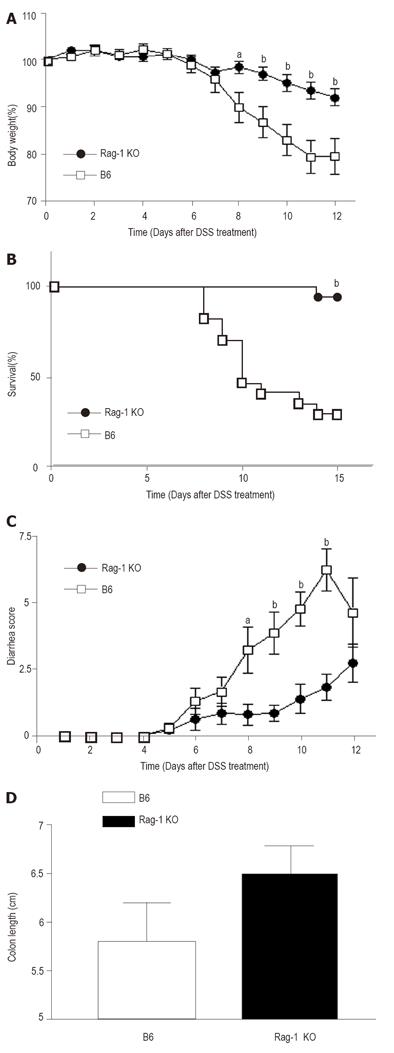
To test the role of lymphocytes in the development of DSS-induced colitis, we administrated various doses of DSS orally into wild-type control and Rag-1 KO mice and investigated the clinical and pathological findings. We could not detect any differences between B6 control and Rag-1 KO mice when treated with high dose (5%) of DSS. However, the progression of colitis significantly slowed down in Rag-1 KO mice treated with low dose (1.5%) of DSS. To evaluate the disease activity, we observed the symptomatic parameters, including body weight loss, survival rate and diarrhea. The control B6 mice administered with oral DSS lost body weight, almost 20 ± 3.79% (n = 10) in 12 d. In contrast, the absence of lymphocyte in Rag-1 KO mice significantly prevented the loss of body weight caused by the treatment of DSS, the decrement being 7.98 ± 1.79% (n = 14, P < 0.005 vs control B6 mice/DSS administration). The differences of body weight began to be statistically significant 8 d after DSS administration and maintained through the experiments (Figure 1A). In addition to weight loss, the survival rates and clinical signs were also different between two groups. At the end of the study, while 65% of control mice died or lost weight more than 20%, only 5.5% (1/18) of Rag-1 KO mice lost weight more than 20% (Figure 1B). Furthermore, the severity of diarrhea after DSS treatment was scored according to the scale described in the Materials and Methods. In control B6 group, DSS caused severe diarrhea, which peaked around day 10, and the average score at day 10 reached the level of 5.545 ± 0.55 (n = 11). In comparison, the grade of diarrhea was improved significantly in Rag-1 KO mice, with the average score of 1.36±0.54 (n = 14, P < 0.0001 vs B6 mice-DSS, Figure 1C). Next, to identify the morphological changes induced by DSS treatment, we examined the changes in the colon. In macroscopic examination, we found that the colons in Rag-1 KO mice treated with DSS were less shortened, although it was not statistically significant (Figure 1D). In addition, macroscopic examination showed fewer intra-abdominal adhesion and less erythematous colons in Rag-1 KO mice (not analyzed statistically).
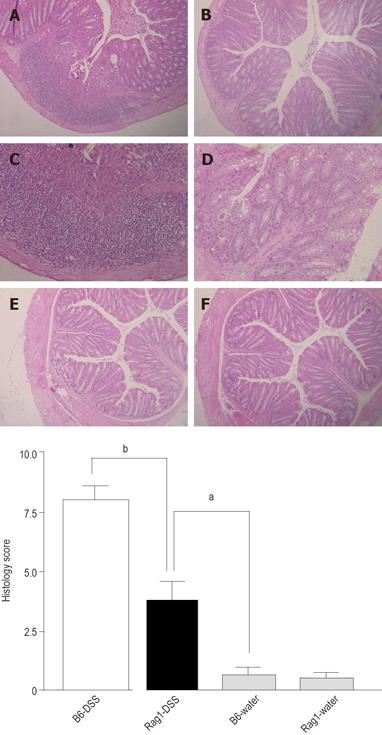
The severity of colonic inflammation was evaluated further by histopathologic examinations. As shown in Figures 2A-2F, wild-type animals often had massive infiltration of mixed inflammatory cells in mucosa and submucosa with epithelial denudation, disruption of mucosal architecture, ulceration, and muscular thickening. In contrast, inflammatory infiltrates were diminished in the colonic mucosa of Rag-1 KO mice, and many areas appeared intact, implying that the effects of lymphocytes in DSS-induced colitis is detrimental. We scored the histological alterations (histology score, HS) as described in the Materials and Methods[9] and demonstrated that the differences in histology between two groups were statistically significant (Figure 2G). The HS in wild-type B6 mice treated with DSS (7.80 ± 0.82) was significantly higher than that in Rag-1 KO mice treated with DSS (3.850±0.63, P = 0.0005). Taken together, our results suggest that lymphocytes play detrimental roles in the development of DSS-induced colitis.
Oral administration of DSS for several days leads to colonic epithelial lesions and acute inflammation characterized by the presence of neutrophils and macrophages within damaged segments. The reason for the deleterious effects of DSS is not well understood, however, epithelial cell toxicity, increased epithelial cell permeability, and macrophage activation have been proposed as potential mechanism[10]. In addition, it has been controversial whether the development of DSS-induced colitis is affected by the presence of lymphocytes[1]. To test the role of lymphocyte in the pathogenesis of DSS-induced colitis, we observed the development and clinical course of DSS-induced colitis in both Rag-1 KO and B6 control mice and clearly showed that the absence of lymphocyte suppressed DSS-induced colitis at low dose DSS stimulation
However, while the overall severities were significantly reduced in Rag-1 KO mice, the inflammatory changes and the shortage of the colons were also observed in Rag-1 KO mice compared to that in wild-type B6 control mice. In addition, we could not detect any differences in clinical courses and morphological changes between Rag-1 KO and B6 control mice when treated with high dose of DSS (5%, data not shown). Taken together, these results suggest that the roles of lymphocytes are dispensable in DSS-induced colitis initiation and considered as an aggravating factor under the presence of weak insults (low dose of DSS)[1]. Our hypothesis is also supported by a previous paper which showed that adoptive transfer of primed T cells from DSS-treated mice by itself did not induced colitis but aggravated DSS-induced colitis, in contrast to the data from TNBS-treated Th1-type colitis model[11].
Given the contribution of lymphocyte to DSS-induced pathology, the data presented here lead to the question of “how”. At the moment, this question can only be answered in general terms by the following suggestions: DSS is known to be directly toxic to colonic epithelial cells and disruption of colonic epithelial barriers allows monocytes-macrophages in lamina propria to enter the activation phase[10] and then macrophages uptake and present foreign peptide into T cells in addition to the secretion of pro-inflammatory cytokines. The activated T cells contribute to DSS-induced pathology as an aggravating factor.
In summary, our results show that lymphocytes play a crucial role in the induction of DSS-induced colitis resembling human ulcerative colitis as aggravating factors.
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Wu M
| 1. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1024] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 2. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] |
| 3. | Axelsson LG, Landström E, Goldschmidt TJ, Grönberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+) -cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3061] [Cited by in RCA: 3165] [Article Influence: 150.7] [Reference Citation Analysis (0)] |
| 5. | Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643-1652. [PubMed] |
| 6. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 927] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 7. | Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695-702. [PubMed] |
| 10. | Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267-278. [PubMed] |
| 11. | Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T. Involvement of CD4+ T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol. 1998;31:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |