Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.298
Revised: June 8, 2005
Accepted: June 18, 2005
Published online: January 14, 2006
AIM: To study the relationship between particularly interesting new cysteine-histidine rich protein (PINCH) expression and clinicopathological factors in Chinese colorectal cancer patients.
METHODS: The expression of PINCH was examined by immumohistochemistry in 141 samples of primary colorectal adenocarcinoma and 92 normal samples of colorectal mucosa. Eighty of the cases had both primary tumour and normal mucosa from the same patients.
RESULTS: PINCH was expressed in the stroma of normal mucosa and tumours. PINCH expression in tumour-associated stroma was increased compared to normal mucosa in both unmatched cases (n = 141, X2 = 85.79, df = 3, P < 0.0001) and matched cases (n = 80, X2 = 45.86, df = 3, P < 0.0001). Among 135 tumours with visible invasive margin, 86 (64%) showed stronger PINCH expression at the invasive margin than in the intratumoural stroma. The frequency of PINCH strong expression in mucinous and signet-ring cell carcinomas was higher (52%) compared to non-mucinous carcinomas (29%, X2 = 5.13, P = 0.02). We did not find that PINCH expression was related to patient’s gender, age, tumour location, tumour size, gross status, histological type, differentiation, invasion depth, lymph node status and Dukes’ stage (P > 0.05).
CONCLUSION: The expression of PINCH was upregulated in colorectal cancers, and especially at the margin of tumours, and further was related to mucinous and signet-ring cell carcinomas. The results suggest that expression of PINCH may be involved in the tumourigenesis and aggressiveness of colorectal cancers.
- Citation: Zhao ZR, Zhang ZY, Cui DS, Jiang L, Zhang HJ, Wang MW, Sun XF. Particularly interesting new cysteine-histidine rich protein expression in colorectal adenocarcinomas. World J Gastroenterol 2006; 12(2): 298-301
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.298
Studies have shown the importance of stromal tissue in regulating the physiological processes of the body. Disruption of stromal-epithelial interactions and cell adhesion alters cellular signalling, which influences proliferation, angiogenesis, differentiation, motility, death, genomic integrity and other phenotype in the cells and tissues[1-3]. Extra-cellular matrix (ECM) adhesion is a fundamental process that controls a variety of cellular processes including cell shape changes and migration. Cell-ECM interactions are mediated by a selective group of membrane and cytoplasmic proteins at the ECM contact sites. Integrin-linked kinase (ILK) is a multidomain protein that plays an important role at ECM adhesion sites [2-7].
PINCH is a LIM domain adapter protein. The PINCH gene is located on chromosome 2q12.2. PINCH and ILK components function as an adaptor protein connecting the growth factor-signalling pathways with the integrin-signalling pathway. It is found in focal adhesions, large cellular complexes that link extracellular matrix to the actin cytoskeleton interacting with ILK and Nck2. PINCH has been implicated as a platform for multiple protein-protein interactions mediating integrin signalling within focal adhesions[4,7-11].
In the present study, the expression of PINCH was immunohistochemically studied in 141 samples of primary colorectal adenocarcinoma and 92 normal mucosa samples. The aims were to investigate the expression of PINCH in normal mucosa and primary tumours in Chinese colorectal cancer patients and to identify the relationship between PINCH expression and clinicopathological variables including patient’s gender, age, tumour location, tumour size, gross status, histological type, grade of differentiation, invasive depth, metastasis in the lymph nodes and Dukes’ stages.
Tumour samples were collected from 141 patients with colorectal adenocarcinoma diagnosed at Department of Pathology, Tangshan Worker’s Hospital, China, between 2000 and 2002. The study also included 92 normal mucosa samples, 80 of which were matched with the tumours, i.e., from the same patients. Normal samples were taken from the margin of the distant resection being histologically free from pre-tumour and tumour. None of the patients received preoperative radiotherapy or chemotherapy. The patient’s gender, age, tumour location, tumour size, gross status, histological type, grade of differentiation, invasive depth, lymph node status and Dukes’ stages were obtained from surgical and pathological records. The mean age of the patients was 56 years (range, 30 - 80 years). Tumours of the caecum, ascending and transverse colon were defined as proximal tumours and tumours of the descending and sigmoid colon and rectum were defined as distal tumours. The mean tumour size was 4.9 cm (range, 0.8 - 20 cm). Tumours were graded as better (well + moderate) and worse (poor) differentiation. All samples were examined by two pathologists.
The preparation, specificity and reliability of the rabbit polyclonal PINCH antibody (obtained kindly from Professor Ann Rearden, Department of Pathology, University of California, La Jolla, CA) used in this study have been described previously [10,11].
The paraffin-embedded tissue sections (5μm) were deparaffinised in xylene and rehydrated in graded ethanol. In order to expose masked epitopes, the sections were boiled in 0.01 mol/L Tris-EDTA buffer (pH 9.0) in a high pressure-cooker, kept at room temperature for 30 min and washed with phosphate-buffered saline (PBS, pH 7.4). The activity of endogenous peroxidase was blocked in 0.5% H2O2 in methanol for 10 min and washed with PBS. The sections were incubated with the 2μg/mL primary PINCH antibody at 4 °C overnight and washed with PBS. The sections were incubated with polymer enhancer for 20 min, then with polymerized HRP-anti mouse/rabbit IgG antibody for 30 min and rinsed in PBS between the incubation steps. After washed with PBS, peroxidase reaction was performed by use of 3,3’-diaminobenzidine for 5 min [Elivision TM plus polyer HRP (mouse/rabbit) IHC kit, Fuzhon Maixin Biology Technology Limited Company, Fuzhou, China]. After counterstained with haematoxylin, the sections were dehydrated and mounted. Normal mucosa and the matched primary tumours were stained in the same run of immunostaining to avoid bias on the pattern and intensity of the staining. Sections known to show strong immunostaining for PINCH were used in each run receiving either the primary antibody or PBS as positive and negative controls. In all the staining procedures, the positive controls showed staining clearly and there was no staining in the negative controls.
PINCH immunostaining was independently examined by two pathologists in a blinded fashion without know-ledge of the clinical and pathological information. To avoid artificial effect, the staining on the margins of sections and areas of poorly presented morphology were not counted. The intensity of PINCH staining in stroma was graded as negative (no positive cells), weak (<5% positive cells), moderate or strong staining. In cases with discrepant results, a consensus score was reached after re-examination.
The chi square method was used to test the relationship between the frequencies of PINCH expression in normal mucosa and tumour as well as between PINCH expression in tumour and clinicopathological variables. All P values cited were two-sided and P<0.05 was considered statistically significant.
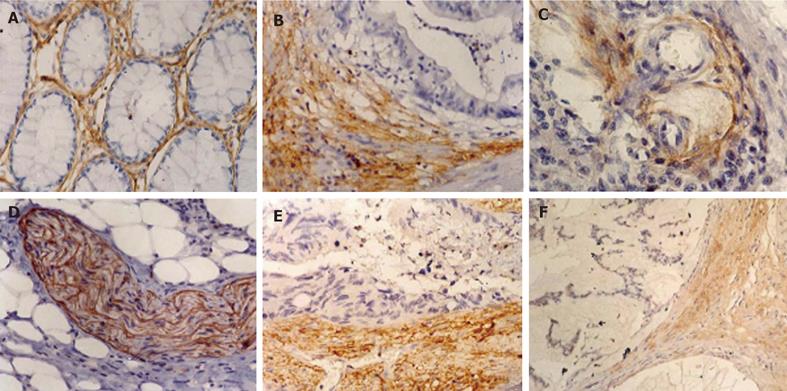
PINCH was expressed in the stroma in both normal mucosa and tumour samples, mainly in cytoplasm of fibroblasts and myofibroblasts, a proportion of endothelial cells in the tumour vasculature and peripheral nerves (Figure 1). Among 135 tumours with visible margin, 86 (64%) showed stronger PINCH expression at the invasive edges than in the intratumoural stroma, 31 (23%) showed opposite evidence, the remained 18 (13%) showed the same staining intensity. There was no PINCH expression in normal epithelial and tumour cells (Figure 1).
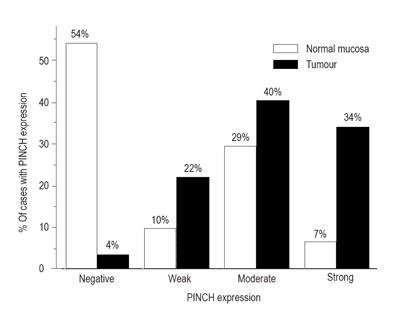
PINCH expression was negative in 50 (54%), weak in 9 (10%), moderate in 27 (29%) and strong in 6 (7%) in 92 normal mucosa samples, and in 5 (4%), 31 (22%), 57 (40%) and 48 (34%) respectively in 141 tumour samples. The expression was significantly increased in tumour samples compared to normal mucosa samples (χ2 = 85.79, df = 3, P < 0.0001, Figure 2). Even in the 80 matched cases of normal mucosa samples (51%, 10%, 33% and 6%) and tumours (5%, 20%, 43% and 33%), the significance was still remained (χ2 = 45.86, df = 3, P < 0.0001).
Since the clinicopathological features of tumours with negative, weak and moderate staining were similar, they were combined as one group (weak group) to compare with strong group in statistical analyses. Table 1 summarises the expression of PINCH in relation to patient’s gender, age, tumour location, tumour size, gross status, histological type, grade of differentiation, invasive depth, lymph node status and Dukes’ stage. The result showed that the frequency of strong PINCH expression was higher in mucinous/signet-ring cell carcinomas (52%) than in non-mucinous carcinomas (29%, χ2 = 5.13, P = 0.02, Table 1). Figure 1 shows a mucinous carcinoma with PINCH expression. The frequency of strong PINCH expression in Dukes C’C + D tumour (45%) tended to be higher than that in Dukes’ A + B tumour (30%, χ2 = 2.65, P = 0.10). It was also shown that patients with lymph node metastasis seemed to have higher PINCH expression (44%) than those without metastasis (30%, χ2 = 2.50, P = 0.11). We did not find other relationships between the expression of PINCH and clinicaopathological variables (P > 0.05, Table 1).
| Variables | n | Strong PINCHn (%) | Weak PINCHn (%) | P |
| Gender | 0.67 | |||
| Male | 71 | 23 (32) | 48 (68) | |
| Female | 70 | 25 (36) | 45 (64) | |
| Age (years) | 0.77 | |||
| ≤56 | 70 | 23 (33) | 47 (67) | |
| 56 | 71 | 25 (35) | 46 (65) | |
| Tumour location | 0.61 | |||
| Proximal | 42 | 13 (31) | 29 (69) | |
| Distal | 99 | 35 (35) | 64 (65) | |
| Tumour size (cm) | 0.64 | |||
| ≤4.9 | 86 | 28 (33) | 58 (67) | |
| >4.9 | 55 | 20 (36) | 35 (64) | |
| Gross status | 0.6 | |||
| Ulcerated | 108 | 38 (35) | 70 (65) | |
| Polypoid/Lager fungating | 33 | 10 (30) | 23 (70) | |
| Histological type | 0.02 | |||
| Non-mucinous | 117 | 34 (29) | 83 (71) | |
| Mucinous/signet-ring cell | 27 | 14 (52) | 13 (48) | |
| Grade of differentiation | 0.59 | |||
| Better | 99 | 33 (33) | 66 (67) | |
| Worse | 42 | 16 (38) | 26 (62) | |
| Lymph node status | 0.11 | |||
| Non-metastasis | 100 | 30 (30) | 70 (70) | |
| Metastasis | 41 | 18 (44) | 23 (56) | |
| Invasive depth | 0.48 | |||
| Intra-bowel wall | 105 | 34 (32) | 71 (68) | |
| Ultra-bowel wall | 36 | 14 (39) | 22 (61) | |
| Dukes’ stage | 0.17 | |||
| A | 52 | 12 (23) | 40 (77) | |
| B | 51 | 19 (37) | 32 (63) | |
| C | 34 | 15 (44) | 19 (56) | |
| D | 4 | 2 (50) | 2 (50) |
In the present study, we observed that PINCH presented in fibroblasts, myofibroblasts, a proportion of endothelial cells of the tumour vasculature and peripheral nerves. The expression of PINCH was especially strong in stroma at the invasive edges of tumours compared to the intratumoural stroma, suggesting that PINCH as a biological factor, may be involved in the angiogenesis and invasiveness of tumour. This evidence may partly explain why strong PINCH expression is associated with a poor prognosis in colorectal cancer patients[12]. In the present study, the frequency of strong PINCH expression was significantly higher in mucinous and signet-ring cell carcinomas than in non-mucinous carcinomas, which may also explain why PINCH expression is related to a poor clinical outcome. Studies demonstrated that patients with mucinous colorectal carcinomas have a worse prognosis than those with non-mucinous carcinomas[13-15], indicating that mucins interfere with immunologic recognition of tumour cells by masking antigenic epitopes with sialic acid residues and inhibiting lymphocyte infiltration[16]. We have previously reported that there is less inflammatory infiltration in colorectal cancer with strong PINCH expression[12].
PINCH is directly associated with ILK and Nck-2 proteins that are downstream effectors of integrin and growth factor signalling. Some of these growth factors, such as PDGF-mediated tumour-stromal interactions are impotant to tumour growth. PINCH is required for ILK localisation to integrin-containing adheren junctions where ILK regulates fibronectin matrix assembly[4,6,7,9-12], suggesting that PINCH protein may increase the upregulated growth factor signalling in stromal cells and a marker for stroma angiogenesis and invasion of tumour cells.
PINCH protein is involved in integrin-mediated cell- ECM interactions, where the different mechanisms or different genetic pathways may develop different histological types of tumour by specific classes of carcinogens. In this context, the expression of PINCH may be associated with the phenotype of epithelial cells in the colorectum. The frequency of K-ras mutation and microsatellite instability is higher in mucinous carcinomas than in non-mucinous carcinomas[17-20]. In contrast, mucinous carcinomas exhibit significantly less p53 mutation and protein[20-23]. These results lead to the hypothesis that K-ras and microsatellite instability may influence mucus production or degradation, resulting in the development of mucinous carcinoma. In contrast to non-mucinous tumours, the development of mucinous carcinomas may be independent from p53 alteration. Thus, PINCH may be another factor involved in the development of mucinous carcinomas.
In conclusion, the expression of PINCH was upregulated in colorectal cancers, and especially at the margin of the tumours. and further was related to mucinous carcinomas. the results suggest that expression of PINCH may be involved in the tumourigenesis and aggressiveness of colorectal cancers.
The authors thank Professor Ann Rearden at the Department of Pathology, University California, CA, USA, for providing the PINCH antibody, and Dr.Yu-Xin Gu for helping in the experiment.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK L- Editor Wu M
| 1. | van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rüdiger M, Schlüter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 370] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1258] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 4. | Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425-2434. [PubMed] |
| 5. | Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1489] [Cited by in RCA: 1435] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 6. | Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607-22610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol. 1999;144:45-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Tu Y, Li F, Wu C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell. 1998;9:3367-3382. [PubMed] |
| 9. | Velyvis A, Yang Y, Wu C, Qin J. Solution structure of the focal adhesion adaptor PINCH LIM1 domain and characterization of its interaction with the integrin-linked kinase ankyrin repeat domain. J Biol Chem. 2001;276:4932-4939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Campana WM, Myers RR, Rearden A. Identification of PINCH in Schwann cells and DRG neurons: shuttling and signaling after nerve injury. Glia. 2003;41:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Wang-Rodriguez J, Dreilinger AD, Alsharabi GM, Rearden A. The signaling adapter protein PINCH is up-regulated in the stroma of common cancers, notably at invasive edges. Cancer. 2002;95:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Gao J, Arbman G, Rearden A, Sun XF. Stromal staining for PINCH is an independent prognostic indicator in colorectal cancer. Neoplasia. 2004;6:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37:1891-1900. [PubMed] |
| 14. | Suma KS, Nirmala V. Mucinous component in colorectal carcinoma--prognostic significance: a study in a south Indian population. J Surg Oncol. 1992;51:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol. 1996;91:2195-2199. [PubMed] |
| 16. | Niv Y. Mucin and colorectal cancer metastasis. Am J Gastroenterol. 1994;89:665-669. [PubMed] |
| 17. | Laurent-Puig P, Olschwang S, Delattre O, Validire P, Melot T, Mosseri V, Salmon RJ, Thomas G. Association of Ki-ras mutation with differentiation and tumor-formation pathways in colorectal carcinoma. Int J Cancer. 1991;49:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Bocker T, Schlegel J, Kullmann F, Stumm G, Zirngibl H, Epplen JT, Rüschoff J. Genomic instability in colorectal carcinomas: comparison of different evaluation methods and their biological significance. J Pathol. 1996;179:15-19. [PubMed] |
| 19. | Messerini L, Vitelli F, De Vitis LR, Mori S, Calzolari A, Palmirotta R, Calabrò A, Papi L. Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol. 1997;182:380-384. [PubMed] |
| 20. | Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol. 1999;14:1057-1061. [PubMed] |
| 21. | Lanza G, Maestri I, Dubini A, Gafa R, Santini A, Ferretti S, Cavazzini L. p53 expression in colorectal cancer: relation to tumor type, DNA ploidy pattern and short-term survival. Am J Clin Pathol. 1996;105:604-612. [PubMed] |
| 22. | Campo E, de la Calle-Martin O, Miquel R, Palacin A, Romero M, Fabregat V, Vives J, Cardesa A, Yague J. Loss of heterozygosity of p53 gene and p53 protein expression in human colorectal carcinomas. Cancer Res. 1991;51:4436-4442. [PubMed] |
| 23. | Hanski C, Tiecke F, Hummel M, Hanski ML, Ogorek D, Rolfs A, Schmitt-Gräff A, Stein H, Riecken EO. Low frequency of p53 gene mutation and protein expression in mucinous colorectal carcinomas. Cancer Lett. 1996;103:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |