Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.214
Revised: May 28, 2005
Accepted: June 2, 2005
Published online: January 14, 2006
AIM: To examine the potency of 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG) as a hepatic heme oxygenase-1 (HO-1) inducer and its regulation in HepG2 cells.
METHODS: Expression of HO-1 and NF-E2-related factor 2 (Nrf2) and activation of mitogen-activated protein (MAP) kinases were analyzed by Western blot, immunofluorescence assay, and flow cytometry. Transfections of HO-1 gene, small interfering RNAs for HO-1 and Nrf2, and dominant-negative gene for MAP/extracellular signal-regulated kinase (ERK) were carried out to dissect the signaling pathways leading to HO-1 expression in HepG 2 cells.
RESULTS: PGG up-regulated HO-1 expression and this expression conferred cytoprotection against oxidative injury induced by t-butyl hydroperoxide. Moreover, PGG induced Nrf2 nuclear translocation, which was found to be an upstream step of PGG-induced HO-1 expression, and ERK activation, of which pathway was involved in PGG-induced Nrf2 nuclear translocation, HO-1 expression and cytoprotection.
CONCLUSION: PGG up-regulates HO-1 expression by stimulating Nrf2 nuclear translocation in an ERK-dependent manner, and HO-1 expression by PGG may serve as one of the important mechanisms for its hepatoprotective effects.
- Citation: Pae HO, Oh GS, Jeong SO, Jeong GS, Lee BS, Choi BM, Lee HS, Chung HT. 1,2,3,4,6-penta-O-galloyl-β-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J Gastroenterol 2006; 12(2): 214-221
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.214
Oxidative stress is the inappropriate exposure to reactive oxygen species (ROS), such as superoxide anion radicals and hydroxyl radicals. High levels of ROS cause damage to cells and are involved in several human pathologies, including liver cirrhosis and fibrosis[1,2]. Therefore, the use of compounds with antioxidant properties may help prevent or alleviate many diseases associated with ROS. Because ROS formation is a naturally occurring process, the mammalian cells have developed several protective mechanisms to prevent ROS formation or to detoxify the ROS. These mechanisms employ molecules called antioxidants as well as protective enzymes[3]. Among the various cytoprotective enzymes, heme oxygenase-1 (HO-1) is recently highlighted by virtue of its hepatoprotective roles[4-6].
The inducible HO-1 is a rate-limiting enzyme in heme catabolism, leading to the formation of carbon monoxide, iron, and biliverdin. Biliverdin is subsequently converted to bilirubin, a potent endogenous anti-oxidant[7]. There is a large body of evidence suggesting that HO-1 plays a key role in maintaining anti-oxidant homeostasis during cellular stress[7-9]. The induction of HO-1 gene is primarily regulated at the transcriptional level, and its inducibility by various inducers is linked to the transcription factor NF-E2-related factor 2 (Nrf2)[9].
Under normal conditions, Nrf2 is sequestered in the cytoplasm by binding to Keep1, an actin-binding protein[10]. Several stimuli cause the disruption of this complex, freeing Nrf2 for translocation to the nucleus and dimerization with basic leucine zipper transcription factors[11,12]. The mechanism by which Nrf2 is liberated from the Keep1-Nrf2 complex remains to be established. However, recent studies have suggested that the Nrf2 nuclear translocation requires the activation of the mitogen-activated protein kinases (MAPKs)[13].
The three major MAPK pathways are represented by kinase cascades leading to activation of extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK)[14]. All three pathways appear to be involved to some extent in HO-1 expression and Nrf2 nuclear translocation in response to diverse stimuli[15-20].
In view of the growing evidence that HO-1 provides hepatoprotection[4-6], HO-1 expression by pharmacological modulator may represent a novel target for therapeutic intervention. Particularly, the identification of a non-cytotoxic inducer of HO-1 may maximize the intrinsic antioxidant potential of cells. Several antioxidants from plant origins have been reported to induce HO-1 expression in a variety of cells, including liver cells, and hence to resist to the oxidative stress[15-17,21-24].
1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG), a bioactive tannin, is wide-spread and can be found in many medicinal plants[25]. Previously, we have demonstrated that PGG is a promising inducer of HO-1[26]. In this study, we aimed at examining the potency of PGG as a hepatic HO-1 inducer and its regulation in HepG2 cells. In addition, we examined the hepatoprotective significance of PGG-induced HO-1 against oxidant toxicity.
HepG2 cells were obtained from ATCC (Manassas, VA) and maintained as monolayer cultures in F12 medium supplemented with 100 mL/L fetal bovine serum (FBS), 1.7 mg/mL sodium bicarbonate, 0.1 unit/mL insulin, 0.5 x minimal essential medium amino acid, 100 units/mL penicillin, and 100 µg/mL streptomycin.
Antibodies to phospho (p)-ERK-1/2, p-JNK-1/2, p-p38, ERK-1/2, JNK-1/2, p38, and Nrf2, and small interfering (si) RNAs for HO-1 and Nrf2 plus siRNA transfection kit were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). FITC-conjugated secondary antibody was purchased from BD PharMingen (San Diego, CA). Antibodies to HO-1, tubulin, and lamin B were obtained form Cell Signaling Technology (Beverly, MA). cDNAs encoding constitutively active (CA) and dominant negative (DN) mutants of MEK1 were kindly provided by Dr. K. Y. Choi (Yonsei University, Seoul, Korea). HO-1 cDNA was a kind gift from Dr. A. M. K. Choi (University of Pittsburgh, Pittsburgh, PA).
PGG was isolated from the root of Paeonia lactiflora, as previously described[27]. U0126 and zinc protoporphyrin IX (ZnPP) were obtained from Promega (Madison, WI) and Porphyrin Products (Logan, UT), respectively. SP600125 was purchased from Calbiochem (San Diego, CA). SB203580 and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Total RNA was isolated by using TRI Reagent™ (Sigma-Aldrich). First-strand cDNAs were synthesized from RNA using ImProm-II RT system (Promega). Then, PCR with Taq DNA polymerase (Promega) was performed for 27 cycles, and PCR products were analyzed by electrophoresis. The primers used for PCR amplification were 5’-ATGGATGATGATATCGCCGCG-3’ and 5’-TCTCCATGTCGTCCCAGTTG-3’ (β-actin, 248 bp), as well as 5'-AAGATTGCCCAGAAAGCCCTGGAC-3' and 5'-AACTGTCGCCACCAGAAAGCTGAG-3' (HO-1, 399 bp).
Cells were solubilized in ice-cold 10 g/L Triton X-100 lysis buffer supplemented with protease and phosphatase inhibitors. After 30 min on ice, the lysates were clarified by centrifugation and the protein concentration was determined. Proteins were resolved with SDS-PAGE, transferred to nitrocellulose membranes, and probed with specific antibodies (diluted 1:1 000), followed by incubation with secondary antibody (diluted 1:5 000) for specific band detection. HO-1 activity was determined at the end of each treatment as described in our previous study[26].
Cells were grown on Lab-Tek II chamber slides. The cells were fixed using formalin and permeabilized with cold acetone. Immunofluorescence was then performed using Nrf2 or HO-1 antibody and FITC-labeled secondary antibody. The cells were stained with DAPI (Sigma-Aldrich) to visualize the nuclei. A Zeiss Axiovert 200 fluorescent microscope fitted with a HV-Deo camera and the appropriate filters were used to capture the fluorescent images.
Cells were washed three times with PBS, fixed with 40 g/L paraformaldehyde and permeabilized with 1 g/L saponin. HO-1 expression was determined by intracellular staining with HO-1 antibody labeling FITC-conjugated secondary antibody. The stained cells were analyzed using a FACSVantage flow cytometry (BD Biosciences, Franklin Lakes, NJ).
Cell toxicity was estimated using the tetrazolium salt reduction test (MTT) after 4-h exposure to t-butyl hydroperoxide (t-Bt-HP; Sigma-Aldrich). After changing culture medium, MTT (5 mg/mL in PBS) was added to a plate. Cells were allowed to incubate for 3 h at 37°C in a atmosphere containing 50 mL/L CO2. After solubilization of deposited MTT with dimethyl sulfoxide, absorbance was measured at 540 nm in a microplate reader.
HepG2 cells were plated in 6-well plates at a density of 1.5 x 105 cells/well. After 24 h of plating, cells were transfected with expression plasmids using Lipofect-AMINE 2000 (Invitrogen), following the manufacturer’s instructions. Briefly, cell culture medium was changed to a fresh medium before each transfection, 0.5 µg of pRSV-β-galactosidase plasmid was co-transfected for transfection efficiency normalization in each transfection, and total amount of DNA transfected in each well was adjusted to 4 µg by using the empty vector pCDNA3.1. Cells were incubated with transfection mixture for 5 h and then cultured in fresh medium for an additional 36 h. Transfection for each siRNA was carried out with siRNA transfection kit (Santa Cruz Biotechnology), following the manufacturer’s instructions. The transfected cells were grown for 36 h.
Results were expressed as means ± SE. Student’s t-test was applied for comparison of the data. P < 0.05 was considered statistically significant.
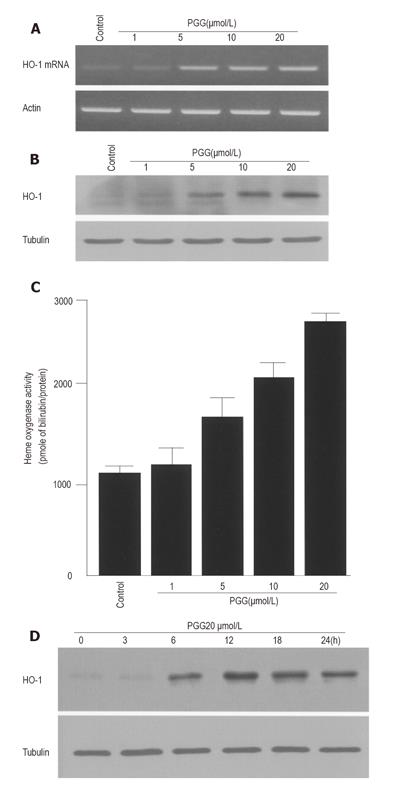
To determine the effects of PGG on HO-1 gene transcription, we used human HepG2 cells, which have been widely used in drug metabolism, chemoprevention and hepatoprotective studies[28]. HO-1 mRNA expression was analyzed by RT-PCR after treatment with PGG (1-20 µmol/L) for 4 h. HO-1 mRNA expression was increased in a dose-dependent manner (Figure 1A). No significant toxicity was found to the cells at these concentrations of PGG (data not shown). To further determine whether PGG could induce HO-1 protein expression, cells were treated with different doses of PGG for 12 h. Treatment with PGG increased HO-1 protein expression in a dose-dependent manner (Figure 1B). In addition, HO activity was also increased in a dose-dependent manner (Figue 1C). In HepG2 cells treated with 20 µmol/L of PGG, HO-1 expression was increased in a time-dependent manner, reaching a maximum at 12 h, followed by a decrease at 24 h (Figure 1D).
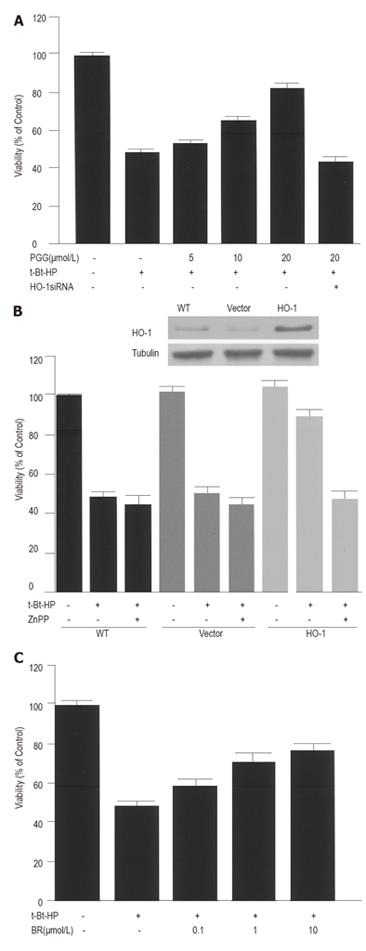
To examine whether HO-1 expression by PGG could cause HepG2 cells to become more resistant to oxidative injury, the cells were pre-treated with medium or PGG (5-20 µmol/L) for 12 h. After changing culture medium, cells were exposed for 4 h to t-Bt-HP, a compound commonly used to induce oxidative stress in biological system[29]. Exposure to 100 µmol/L of t-Bt-HP resulted in a marked reduction of cell viability. Pre-incubation with PGG for 12 h diminished t-Bt-HP toxicity in a dose-dependent manner and increased cell viability by 80% (Figure 2A). Cytoprotection by PGG was not detectable when the cells were transiently transfected with HO-1 siRNA (Figure 2A). Moreover, a similar cytoprotective effect was observed when the cells were transfected with HO-1 gene (Figure 2B). Blockage of the enzyme activity by ZnPP, a specific HO inhibitor, abolished HO-1-mediated cytoprotection (Figue 2B), suggesting that HO-1 metabolites could be of functional relevance of the observed protective action. Indeed, exogenously added bilirubin (0.1-10 µmol/L), one of three HO-1 metabolites, produced a substantial reduction of t-Bt-HP toxicity (Figure 2C). HO-1 siRNA and ZnPP alone had no significant effect on cell viability under these conditions (data not shown).
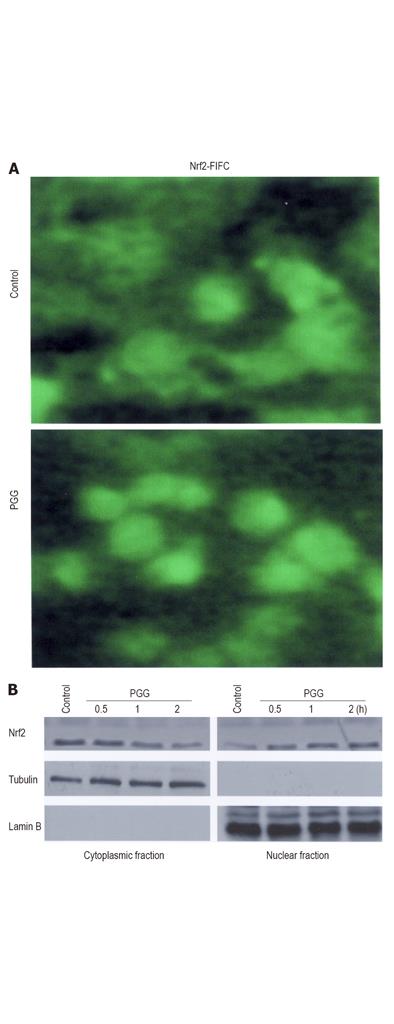
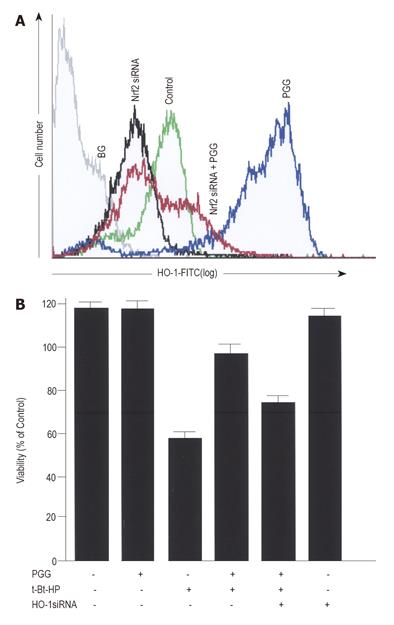
In the mechanism of HO-1 expression, nuclear translocation of activated Nrf2 is an important upstream step[9,30]. To investigate whether PGG could induce Nrf2 translocation in HepG2 cells, an immunofluorescence assay was used to detect the distribution of Nrf2 in the cells treated with medium or PGG (Figure 3A). Approximately 20 µmol/L PGG was chosen to further determine the effects of PGG, because PGG at this concentration showed no significant toxicity (data not shown), caused a maximal increase in HO-1 expression (Figure 1) and also conferred a maximal cytoprotection (Figure 2). In untreated HepG2 cells, Nrf2 fluorescence was found to be distributed throughout the cells including cytoplasm and nucleus[15]. After treatment with PGG for 2 h, Nrf2 fluorescence was primarily concentrated in the nuclei (Figure 3A). To further confirm the Nrf2 nuclear translocation by PGG, cells were incubated with PGG for about 2 h. Using Western blot, Nrf2 proteins in the cytoplasm and nuclear compartments of the cells were analyzed. After treatment with PGG, Nrf2 protein in the cytoplasm was decreased, and Nrf2 protein in the nucleus was markedly increased (Figure 3B). In addition, PGG-induced HO-1 expression was significantly reduced by transient transfection with Nrf2 siRNA (Figure 4A), as quantified by a flow cytometry, and cytoprotection afforded by PGG was effectively abolished by Nrf2 siRNA (Figure 4B).
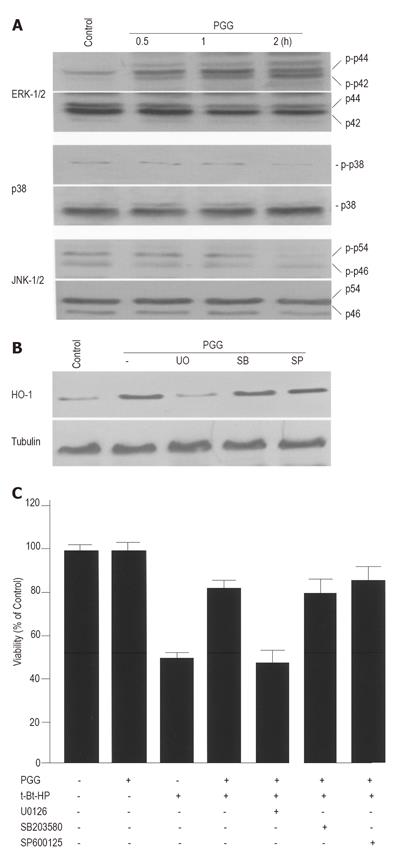
MAPKs have been reported to participate in the regulation of HO-1 expression[15-20]. We examined the effects of PGG on MAPK activities in HepG2 cells. The cells were treated with medium or 20 µmol/L PGG for about 2 h, and cell extracts were analyzed for phosphorylated and total MAPKs by Western blot. While the phosphorylated forms of all three MAPKs could be detectable even in the untreated cells, only the phosphorylated form of ERK was significantly increased by PGG (Figure 5A). Increased phosphorylation of ERK was detected within 30 min after treatment with PGG, and the amount of this kinase remained above the basal level for about 2 h. To address the role of individual MAPK pathway in HO-1 expression by PGG, we examined the effects of U0126, SB203580 and SP600125, specific inhibitors for ERK, p38 and JNK pathways, respectively, on HO-1 expression. Whereas inhibitor of either p38 or JNK had no effect on PGG-induced HO-1 expression, inhibitor for ERK MAPK pathway significantly reduced HO-1 expression (Figure 5B). In addition, we examined the effects of three MAPK inhibitors on PGG-induced cytoprotection. As expected, inhibitor for ERK MAPK pathway abolished PGG-induced cytoprotection, but inhibitors for p38 or JNK MAPK pathways did not (Figure 5C). The inhibitor for ERK alone had no significant toxicity under our experimental conditions (data not shown).
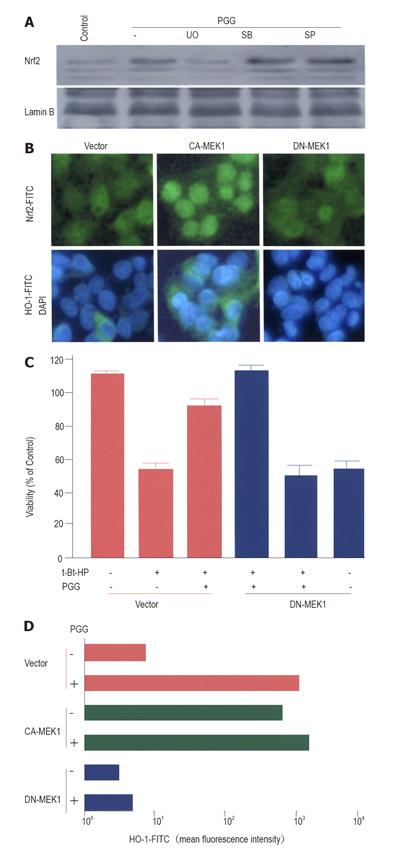
It has been reported that MAPK-directed phosphorylation is a requirement for Nrf2 nuclear translocation in HepG2 cells[31-33]. Thus, we examined whether MAPK pathway could be involved in the process by which PGG caused Nrf2 nuclear translocation. As shown in Figure 6A, inhibitor for ERK MAPK pathway, but not for p38 or JNK MAPK pathways, blocked PGG-induced Nrf2 nuclear accumulation. This was similar to the role of ERK inhibitor in PGG-induced HO-1 expression (Figure 5B) and cytoprotection (Figure 5C). To further evaluate the role of the ERK activation in PGG-induced Nrf2 nuclear translocation and HO-1 expression, we transfected DN-MEK1 and CA-MEK1 genes into HepG2 cells to selectively inhibit or activate the ERK pathway. In HepG2 cells, CA-MEK1 expression itself activated Nrf2 nuclear translocation and also HO-1 expression (Figure 6B). Moreover, PGG-induced cytoprotection (Figure 6C) and HO-1 expression (Figure 6D) were not observed when the cells were transfected with DN-MEK1 gene.
We previously reported that PGG, one of bioactive tannins, induced HO-1 expression[26]. In this study, we aimed to examine the potency of PGG as a hepatic HO-1 inducer and its regulation in HepG2 cells. Our results showed that PGG induced HO-1 expression via stimulating Nrf2 nuclear translocation in an ERK-dependent manner, thereby exerting hepatoprotective effects.
The cytoprotective properties of antioxidants have been partially ascribed to their ability to induce cytoprotective enzymes. Among the various cytoprotective enzymes, HO-1 expression has been considered to be an adaptive and beneficial response to oxidative stress in a wide variety of cells[4-9]. Here, we demonstrated that PGG at non-cytotoxic concentrations increased HO-1 mRNA and protein expressions as well as HO activity in HepG2 cells (Figure 1). The increase of HO-1 expression by PGG conferred cytoprotection against t-Bt-HP-induced oxidative stress(Figure 2A), which was clearly confirmed by transfection of either HO-1 siRNA (Figure 2A) or HO-1 gene (Figure 2B). We also demonstrated that cytoprotection afforded by HO-1 was highly dependent on its enzyme activity (Figure 2B) and bilirubin, one of its enzymatic products (Figure 2C). Our results suggested that PGG-induced HO-1 expression might serve as one of the important mechanisms for its hepatoprotective effects.
While the induction of HO-1 has been extensively reported and is known to be regulated primarily at the level of gene transcription, the molecular mechanism(s) underlying this response is poorly understood. However, recent evidences have implicated Nrf2 in inducer-dependent activation of the HO-1 gene[9,30]. In our study, we found that PGG could activate Nrf2 in HepG2 cells. PGG increased not only Nrf2 nuclear translocation (Figure 3A), but also Nrf2 nuclear accumulation (Figure 3B). Using Nrf2 siRNA transfection method, we could show that the full expression of HO-1 by PGG required Nrf2 nuclear accumulation (Figure 4A). Along with this result, cytoprotection afforded by PGG was reduced by transient transfection with Nrf2 siRNA (Figure 4B). These results suggested that Nrf2 nuclear translocation might play a key role in PGG-induced HO-1 expression and cytoprotection.
MAPK pathways have been reported to be involved in HO-1 expression[15-20] and also in Nrf2-dependent transcription[31-33]. Our experiments designed to determine a possible role of MAPK pathways in PGG-induced HO-1 expression showed that PGG activated ERK pathway (Figure 5A). Additionally, the use of specific inhibitors for MAPK pathways confirmed the involvement of ERK, but not of p38 or JNK pathways, in PGG-induced HO-1 expression (Figure 5B) and cytoprotection (Figure 5C). These results suggested that ERK MAPK pathway might be important for PGG-induced HO-1 expression.
In light of our finding that ERK inhibitor blocked PGG-induced Nrf2 nuclear accumulation (Figure 6A), it is interesting to speculate that ERK pathway may play a role in regulating PGG-induced Nrf2 nuclear translocation. Our hypothesis is that addition of PGG may increase ERK activation in HepG2 cells, somehow to subsequently stimulate Nrf2 nuclear translocation. Increased Nrf2 in the nucleus may increase its DNA-binding activity, followed by HO-1 gene transcription eventually leading to HO-1 expression. This was confirmed by the transfection of HepG2 cells with CA-MEK1 or DN-MEK1 genes to selectively activate or inhibit the ERK pathway. Activation of the ERK pathway by CA-MEK1, even without further stimulation, was able to induce Nrf2-dependent HO-1 expression (Figure 6B). Blockage of the ERK pathway by DN-MEK1 abolished PGG-induced cytoprotection (Figure 6C) and HO-1 expression (Figure 6D). It is interesting that ERK activation is required for Nrf2 nuclear translocation during glutamate cysteine ligase modulatory gene expression by the antioxidant pyrrolidine dithiocarbamate in HepG2 cells[32,33].
In summary, the antioxidant PGG can induce hepatic HO-1 expression in HepG2 cells, and this expression confers hepatoprotection against oxidative injury. PGG also induces Nrf2 nuclear translocation, which is an upstream step of PGG-induced HO-1 expression, and activates ERK MAPK phosphorylation. ERK MAPK pathway is involved in PGG-induced Nrf2 nuclear translocation, HO-1 expression, and hepatoprotection. Thus, we speculate that PGG may be developed as a hepatic HO-1 inducer for therapeutic purposes. Despite the fact that up-regulation of HO-1 is sufficient to produce many beneficial outcomes in a variety of stressful condition[4-9], we do not exclude the possibility that PGG will stimulate the expression of other defensive enzymes, probably depending on Nrf2 nuclear translocation, and that cellular and tissue protection will be achieved by virtue of the concerted action of the multiple pathways being activated.
We gratefully acknowledge Dr. H. Oh for the isolation and purification of PGG, Dr. Augustine M. K. Choi for the kind gift of HO-1 gene, and Dr. K. Y. Choi for the gifts of DN-MEK1 and CA-MEK1 genes.
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Wu M
| 1. | Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Wu J, Zern MA. NF-kappa B, liposomes and pathogenesis of hepatic injury and fibrosis. Front Biosci. 1999;4:D520-D527. [PubMed] |
| 3. | Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000;15:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004;11:1545-1561. [PubMed] |
| 6. | Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 411] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 968] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 8. | Choi BM, Pae HO, Kim YM, Chung HT. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 704] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 11. | Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 384] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Numazawa S, Yoshida T. Nrf2-dependent gene expressions: a molecular toxicological aspect. J Toxicol Sci. 2004;29:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Edmunds JW, Mahadevan LC. MAP kinases as structural adaptors and enzymatic activators in transcription complexes. J Cell Sci. 2004;117:3715-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Gong P, Hu B, Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 812] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 18. | Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun. 2003;307:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 247] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AM, Burow ME, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694-27702. [PubMed] |
| 21. | Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J Pharmacol Exp Ther. 2005;312:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 2004;6:811-818. [PubMed] |
| 23. | Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Lin HC, Cheng TH, Chen YC, Juan SH. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology. 2004;71:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Chen WJ, Lin JK. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J Biol Chem. 2004;279:13496-13505. |
| 26. | Choi BM, Kim HJ, Oh GS, Pae HO, Oh H, Jeong S, Kwon TO, Kim YM, Chung HT. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose protects rat neuronal cells (Neuro 2A) from hydrogen peroxide-mediated cell death via the induction of heme oxygenase-1. Neurosci Lett. 2002;328:185-189. [PubMed] |
| 27. | Lee SJ, Lee HM, Ji ST, Lee SR, Mar W, Gho YS. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. 2004;208:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Majano PL, Medina J, Zubía I, Sunyer L, Lara-Pezzi E, Maldonado-Rodríguez A, López-Cabrera M, Moreno-Otero R. N-Acetyl-cysteine modulates inducible nitric oxide synthase gene expression in human hepatocytes. J Hepatol. 2004;40:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1002] [Cited by in RCA: 986] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 30. | Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071-26078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1063] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 31. | Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum YS, Han J, Gallo MA, Kong AN. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052-23060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003;73:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |