Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.187
Revised: June 28, 2005
Accepted: July 15, 2005
Published online: January 14, 2006
Lactose malabsorption is a very common condition characterized by intestinal lactase deficiency. Primary lactose malabsorption is an inherited deficit present in the majority of the world’s population, while secondary hypolactasia can be the consequence of an intestinal disease. The presence of malabsorbed lactose in the colonic lumen causes gastrointestinal symptoms. The condition is known as lactose intolerance. In patients with lactase nonpersistence, treatment should be considered exclusively if intolerance symptoms are present. In the absence of guidelines, the common therapeutic approach tends to exclude milk and dairy products from the diet. However, this strategy may have serious nutritional disadvantages. Several studies have been carried out to find alternative approaches, such as exogenous β-galactosidase, yogurt and probiotics for their bacterial lactase activity, pharmacological and non pharmacological strategies that can prolong contact time between enzyme and substrate delaying gastrointestinal transit time, and chronic lactose ingestion to enhance colonic adaptation. In this review the usefulness of these approaches is discussed and a therapeutic management with a flow chart is proposed.
- Citation: Montalto M, Curigliano V, Santoro L, Vastola M, Cammarota G, Manna R, Gasbarrini A, Gasbarrini G. Management and treatment of lactose malabsorption. World J Gastroenterol 2006; 12(2): 187-191
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.187
Lactose malabsorption is a very common condition characterized by lactase deficiency, an enzyme occurring in the brush border membrane of the intestinal mucosa that hydrolyzes lactose to its components galactose and glucose [1]. High concentrations of this enzyme are physiologically present in neonates. Post weaning, a genetically programmed and irreversible reduction of its activity occurs in the majority of the world’s population [2] which results in primary lactose malabsorption. Secondary hypolactasia can be the consequence of any condition that damages the small intestinal mucosa brush border or significantly increases the gastrointestinal transit time. Thus, secondary hypolactasia is transient and reversible [3].
The presence of malabsorbed lactose in the colonic lumen does not necessarily result in gastrointestinal symptoms. Only when lactose malabsorption is associated with clinical manifestations as bloating, flatulence, abdominal pain and diarrhea, “lactose intolerance” occurs [4].
Diagnosis of lactose intolerance is often made on a clinical basis and in response to an empirical trial of dietary lactose avoidance. A number of methods are available to diagnose lactose malabsorption [4-8]. The lactose breath hydrogen test is nowadays considered a very simple and useful test in subjects with suspected lactose malabsorption. Undigested lactose is fermented by the colonic microflora with production of hydrogen detectable in pulmonary excretion [6]. Direct lactase enzyme activity performed on small intestinal tissue biopsy samples may be utilized, but it is an invasive procedure and its reliability can be low because disaccharidase activity in a small biopsy specimen does not necessarily reflect the jejunal activity as a whole [4]. Recent evidence suggests that a genetic test of the -13910 C/T polymorphism can be used as the first stage screening test for adult-type hypolactasia [9,10].
In patients with lactase nonpersistence, treatment is considered exclusively in the presence of intolerance symptoms [11]. In the absence of guidelines, the common therapeutic approach tends to exclude milk and dairy products from the diet. However, this strategy may have serious nutritional disadvantages, chiefly for reduced intake of substances such as calcium, phosphorus and vitamins, and may be associated with decreased bone mineral density [12,13]. To overcome these limits, several studies have been carried out to find alternative approaches, such as exogenous β-galactosidase, yogurt and probiotics for their bacterial lactase activity, pharmacological and non pharmacological strategies that can prolong contact time between enzyme and substrate delaying gastrointestinal transit time, and chronic lactose ingestion to enhance colonic adaptation.
Enzyme-replacement therapy with microbial exogenous lactase (obtained from yeasts or fungi) represents a possible strategy for primary lactase deficiency. Enzymes can be added in a liquid form to milk before its consumption or administered in a solid form (capsules or tablets) together with milk and dairy products. Several studies were conducted adding the soluble enzyme to milk some hours before its consumption, thus obtaining a “preincubated milk” [9,14-17]. This strategy is effective in reducing both H2 breath excretion and subjective manifestations of discomfort after milk ingestion. However, these trials were carried out on relatively small series populations. They were not placebo-controlled, and results were not comparable since there was a lack of homogeneity in patient subsets. Furthermore, preincubated milk was not considered practical because of the necessity to add the enzyme some hours before its consumption. The low-lactose milk is a preincubated milk in which the lactose is already pre-hydrolyzed; this product is commercially available but not distributed everywhere (i.e. restaurant, cafeterias, etc). To obviate these problems, several studies have been carried out to show the effectiveness of replacement therapy even when lactase is administered at mealtime [18-22].
In a recent double-blind, placebo-controlled crossover study in 30 intolerant subjects with lactose malabsorption, we showed that both “preincubated milk” and milk treated at mealtime are similarly able to significantly reduce H2 excretion and symptom score, suggesting that enzymes can be used at mealtime because of its better practicality [23].
While the efficacy of liquid exogenous lactase in reducing both H2 excretion and symptoms is widely emphasized, results about the exact rate of efficacy are somehow discordant. Several factors may justify these discrepancies. The different enzyme origin can play a role. It is well-known that, at the same dose, enzymes obtained from different microorganisms have different efficacy in hydrolyzing lactose. In fact, comparative studies have already shown the higher efficacy of lactase derived from K. lactis than that of the enzyme obtained from A. niger [5,19]. The contribution of residual intestinal mucosal lactase activity should also be considered to explain the variation in intolerance symptoms experienced by lactose malabsorbers. The results of non crossover studies without considering intra-individual variables can be biased, if the residual intestinal mucosal enzyme activity is ignored. Moreover, the different dosage of the enzyme could be another influencing factor. In fact, it is known that a close relationship exists between the amount of lactose to be hydrolyzed and the enzyme units required [5,21]. Moreover, the stomach pH and bile salt concentrations could influence the efficacy of exogenous lactase.
Solid lactase preparations, in capsules and tablets, are commercially available alternatives for enzyme-replacement therapy. Several studies have investigated and confirmed their efficacy [25-26]. However, comparative studies have shown that these preparations are more expensive and significantly less effective than prehydrolyzed milk probably due to the enzyme gastric inactivation [11,17]. Their use can be suggested for solid dairy products.
Therapy compliance with β-galactosidase is assured by good palatability [16,21,23] though there are some reported taste alterations [27]. The safety of lactase preparations has recently been confirmed [28]. In conclusion, the addition of exogenous lactase, especially at mealtime, seems to be effective, practical and with no side effects.
It is well-known that fermented milk products improve lactose digestion and symptoms of intolerance in lactose maldigesters [17,29,30]. Onwulata et al [17] demonstrated that commercially available plain yogurt is as effective in reducing H2 and symptoms as prehydrolyzed milk. The use of fermented milk is based on the presence of endogenous lactase activity of yogurt microorganisms. Yogurt is made of milk incubated mainly with two species of lactic acid bacteria, L. bulgaricus and S. thermophilus [29,31]. These microorganisms participate in lactose hydrolysis both during fermentation processes and after lactose ingestion [32-34]. It has been calculated that fermentation decreases lactose content by approximately 25-50% [33-34]. At the same time, this process results in an acid taste and just for this tartness several subjects dislike yogurt. To overcome this limit, the addition of high concentrations of viable L. acidophilus to cold milk has been proposed as an alternative to yogurt, obtaining an unfermented milk (sweet acidophilus milk). However, in a comparative crossover study carried out in eleven lactose malabsorbers, Payne et al [5] first demonstrated that sweet acidophilus milk does not reduce H2 excretion and symptoms significantly as milk treated with a commercial lactase preparation. Further studies have confirmed the inadequate effectiveness of sweet acidophilus milk [17,33,35]. To explain these findings, it has been proposed that bacterial lactase in sweet acidophilus milk is insufficient to show a measurable effect or is not easily available in the intestinal lumen, becoming accessible only if disruption of the cell membrane occurs [30,33]. In fact, the cell membrane structures of lactic acid bacteria play a key role in the availability of β-galactosidase [17,36]. Lin et al [37] compared L. bulgaricus and L. acidophilus, two termophilic bacteria with similar β-galactosidase activity, bile sensitivity and active transport system for lactose, while differing in the resistance of the cell wall membrane structures, as shown by sonication time for maximum β-galactosidase activity measurements. By measuring H2 excretion and clinical score, they found that L. bulgaricus is a better choice for manufacturing non fermented milk products, because the cell wall membrane structures of L. bulgaricus are less tough than those of L. acidophilus, with a consequent better ability to release the enzyme.
To effectively release β-galactosidase, bacteria need an intact cell wall as mechanical protection of the enzyme during gastric passage and against the action of bile [38]. It was demonstrated that gastric acid degrades bacterial lactase activity in 20-60 min [39]. However, the association of L. acidophilus BG2F04 with omeprazole does not result in reduced hydrogen production and gastrointestinal symptoms are not improved after lactose ingestion with respect to lactobacilli without it [40]. These results could have been due to the selected lactic bacteria. In fact, it is well-known that lactobacilli behave differently depending on the species [41,42]. As recently demonstrated, administration of the multiprobiotic product VSL3 cannot reduce H2 excretion and clinical score [43]. Further investigations are necessary to clarify the probiotics role in lactose intolerance therapy, also considering their well-known beneficial effects on intestinal functions, gas metabolism and motility [38].
The bacterial β-galactosidase activity of yogurt is considered to be the main factor responsible for improving lactose digestion; its greater osmolality and energy density can also play a role [38]. Yogurt delays gastric emptying and intestinal transit causing slower delivery of lactose to the intestine, thus optimizing the action of residual β-galactosidase in the small bowel and decreasing the osmotic load of lactose[3,38].
Leichter et al [44] showed that full-fat milk (high energy milk) increase lactose tolerance compared to skimmed milk and aqueous lactose solution. It has been reported that fat improves carbohydrate absorption slowing down gastric emptying and intestinal transit time with a consequent prolongation of contact time between enzyme and substrate [45-47]. Other studies have not confirmed these results [48,49]. In particular, Vesa et al [49] have proved that the ingestion of high energy milk delays more significantly gastric emptying than half-skimmed milk but it does not result in a significant improvement in lactose tolerance. To improve lactose digestion by delaying gastric emptying, co-ingestion of food together with dairy products has also been proposed, and it has been demonstrated that lactose is better tolerated when taken with other foods [19,50].
Pharmacological approaches that can modify gastric emptying and intestinal transit have also been considered. A double-blind randomized cross-over placebo-controlled study [51] evaluated the effect of propantheline and metoclopramide on lactose digestion, and found that propantheline-induced prolongation of gastric emptying improves lactose tolerance as measured by reduced symptoms and H2 breath concentration compared to placebo or metoclopramide. Loperamide has been proposed for its activity on oral-cecal transit time. Szilagy et al [52] demonstrated that loperamide improves H2 excretion and symptoms when administered together with milk. Later on, the authors concluded that the use of loperamide is not advisable because of its side-effects and high cost [53].
Lactase is a non inducible enzyme [1], but it was also reported that continuous lactose consumption decreases hydrogen excretion and the severity of gastrointestinal symptoms [54-58]. Decreased hydrogen excretion is not necessarily the consequence of increased lactose digestion but can depend on adaptative phenomena. This “adaptation” is associated with changes in gut microflora as well as in some colonic functions and features. The increased microbial β-galactosidase activity is one of the hypothesized mechanisms. Hertzler et al [59] showed that fecal β-galactosidase activity is increased after daily milk feeding for 10 d. However, the lower H2 production is not related to higher levels of lactase in the existing flora. It has been suggested that changes in the intestinal microflora could decrease hydrogen production and/or increase intestinal gas consumption [60]. Hill et al [61] have proved that malabsorbed lactose enhances the fermentation ability of bifidobacteria and other lactic acid bacteria which can metabolize lactose without hydrogen production. Perman et al [62] hypothesized that the reduction of colonic pH due to the fermentation of malabsorbed lactose affects bacterial metabolism and inhibits hydrogen production. The placebo effect has been suggested as an additional factor to explain the adaptation in response to continuous lactose ingestion. In a controlled double-blind study, Briet et al [59] have demonstrated increased fecal β-galactosidase, reduced H2 excretion and improved symptoms after lactose ingestion for 13 d. However, in the control group with sucrose, no sign of metabolic adaptation could be found except for a reduced clinical score, suggesting a placebo effect.
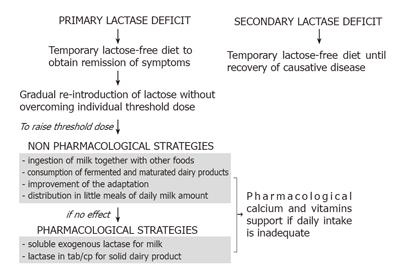
We suggest a flow chart for the therapeutic management of lactose malabsorption considering data gathered from literature and our personal experience (Figure 1). We underline that not all subjects with lactase deficit have to be treated, but just symptomatic ones, since there are no known adverse effects of lactose maldigestion other than acute gastrointestinal symptoms [11]. Furthermore, most lactose-intolerant people can ingest 12 g/d of lactose (equivalent to one cup of milk), without experiencing adverse symptoms [63-64].
It is necessary to distinguish between primary and secondary lactase deficit. In the secondary form a temporary lactose-free diet is necessary only until a complete recovery of the causative pathological condition is obtained. Lactose breath test can be advised to verify the recovered enzymatic activity. In primary hypolactasia, a different therapeutic strategy should be considered because of the irreversibility of the condition. Initially, a temporary avoidance of milk and dairy products from the diet should be indicated to obtain symptom remission. Subsequently, we suggest a gradual re-introduction of dairy products considering the individual threshold dose, to assure an adequate intake of essential nutritional substances. In order to raise the threshold dose, some non-pharmacological and pharmacological strategies should be considered. Changes in dietary habits, as the ingestion of milk together with other foods such as brioches, cookies and cake, and the consumption of fermented and matured dairy products can assure an adequate milk intake while preventing the onset of intolerance symptoms. Chronic consumption of milk seems to be useful to favorite the adaptation. Considering the dose-dependent lactose absorption, we suggest the distribution of the daily milk amount in small meals. If these strategies fail to reduce lactose intolerance, some pharmacological therapies are available. The addition of exogenous lactase in a liquid form to milk at mealtime has been demonstrated effective and practical. Pre-hydrolyzed milk preparations (i.e. on sale low-lactose content milk) could also be advisable. β-galactosidase tablets or capsules are suitable only for consumption of solid dairy products. Based on the literature data no other pharmacological strategies can be suggested. A pharmacological support of calcium and vitamins is required independently of the chosen therapeutic approach if the daily intake of dairy products is not assured.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Li HY
| 1. | Gilat T, Russo S, Gelman-Malachi E, Aldor TA. Lactase in man: a nonadaptable enzyme. Gastroenterology. 1972;62:1125-1127. [PubMed] |
| 2. | Wang Y, Harvey CB, Hollox EJ, Phillips AD, Poulter M, Clay P, Walker-Smith JA, Swallow DM. The genetically programmed down-regulation of lactase in children. Gastroenterology. 1998;114:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Labayen I, Forga L, González A, Lenoir-Wijnkoop I, Nutr R, Martínez JA. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001;15:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Shaw AD, Davies GJ. Lactose intolerance: problems in diagnosis and treatment. J Clin Gastroenterol. 1999;28:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Rosado JL, Solomons NW, Lisker R, Bourges H. Enzyme replacement therapy for primary adult lactase deficiency. Effective reduction of lactose malabsorption and milk intolerance by direct addition of beta-galactosidase to milk at mealtime. Gastroenterology. 1984;87:1072-1082. [PubMed] |
| 6. | Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Vonk RJ, Lin Y, Koetse HA, Huang C, Zeng G, Elzinga H, Antoine J, Stellaard F. Lactose (mal)digestion evaluated by the 13C-lactose digestion test. Eur J Clin Invest. 2000;30:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Swagerty DL, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845-1850. [PubMed] |
| 9. | Rasinperä H, Savilahti E, Enattah NS, Kuokkanen M, Tötterman N, Lindahl H, Järvelä I, Kolho KL. A genetic test which can be used to diagnose adult-type hypolactasia in children. Gut. 2004;53:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Nilsson TK, Johansson CA. A novel method for diagnosis of adult hypolactasia by genotyping of the -13910 C/T polymorphism with Pyrosequencing technology. Scand J Gastroenterol. 2004;39:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Suarez FL, Savaiano DA, Levitt MD. Review article: the treatment of lactose intolerance. Aliment Pharmacol Ther. 1995;9:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Solomons NW, Guerrero AM, Torun B. Effective in vivo hydrolysis of milk lactose by beta-galactosidases in the presence of solid foods. Am J Clin Nutr. 1985;41:222-227. [PubMed] |
| 13. | Di Stefano M, Veneto G, Malservisi S, Cecchetti L, Minguzzi L, Strocchi A, Corazza GR. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology. 2002;122:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Reasoner J, Maculan TP, Rand AG, Thayer WR. Clinical studies with low-lactose milk. Am J Clin Nutr. 1981;34:54-60. [PubMed] |
| 15. | Rask Pedersen E, Jensen BH, Jensen HJ, Keldsbo IL, Hylander Møller E, Nørby Rasmussen S. Lactose malabsorption and tolerance of lactose-hydrolyzed milk. A double-blind controlled crossover study. Scand J Gastroenterol. 1982;17:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Nielsen OH, Schiøtz PO, Rasmussen SN, Krasilnikoff PA. Calcium absorption and acceptance of low-lactose milk among children with primary lactase deficiency. J Pediatr Gastroenterol Nutr. 1984;3:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Onwulata CI, Rao DR, Vankineni P. Relative efficiency of yogurt, sweet acidophilus milk, hydrolyzed-lactose milk, and a commercial lactase tablet in alleviating lactose maldigestion. Am J Clin Nutr. 1989;49:1233-1237. [PubMed] |
| 18. | Payne DL, Welsh JD, Manion CV, Tsegaye A, Herd LD. Effectiveness of milk products in dietary management of lactose malabsorption. Am J Clin Nutr. 1981;34:2711-2715. [PubMed] |
| 19. | Solomons NW, Guerrero AM, Torun B. Dietary manipulation of postprandial colonic lactose fermentation: II. Addition of exogenous, microbial beta-galactosidases at mealtime. Am J Clin Nutr. 1985;41:209-221. [PubMed] |
| 20. | Barillas C, Solomons NW. Effective reduction of lactose maldigestion in preschool children by direct addition of beta-galactosidases to milk at mealtime. Pediatrics. 1987;79:766-772. [PubMed] |
| 21. | Lin MY, Dipalma JA, Martini MC, Gross CJ, Harlander SK, Savaiano DA. Comparative effects of exogenous lactase (beta-galactosidase) preparations on in vivo lactose digestion. Dig Dis Sci. 1993;38:2022-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Corazza GR, Benati G, Sorge M, Strocchi A, Calza G, Gasbarrini G. beta-Galactosidase from Aspergillus niger in adult lactose malabsorption: a double-blind crossover study. Aliment Pharmacol Ther. 1992;6:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Montalto M, Nucera G, Santoro L, Curigliano V, Vastola M, Covino M, Cuoco L, Manna R, Gasbarrini A, Gasbarrini G. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr. 2005;59:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Lami F, Callegari C, Tatali M, Graziano L, Guidetti C, Miglioli M, Barbara L. Efficacy of addition of exogenous lactase to milk in adult lactase deficiency. Am J Gastroenterol. 1988;83:1145-1149. [PubMed] |
| 25. | DiPalma JA, Collins MS. Enzyme replacement for lactose malabsorption using a beta-D-galactosidase. J Clin Gastroenterol. 1989;11:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Biller JA, King S, Rosenthal A, Grand RJ. Efficacy of lactase-treated milk for lactose-intolerant pediatric patients. J Pediatr. 1987;111:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 294] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Flood MT, Kondo M. Toxicity evaluation of a beta-galactosidase preparation produced by Penicillium multicolor. Regul Toxicol Pharmacol. 2004;40:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. 2004;80:245-256. [PubMed] |
| 30. | Hove H, Nørgaard H, Mortensen PB. Lactic acid bacteria and the human gastrointestinal tract. Eur J Clin Nutr. 1999;53:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Bourlioux P, Pochart P. Nutritional and health properties of yogurt. World Rev Nutr Diet. 1988;56:217-258. [PubMed] |
| 32. | Kolars JC, Levitt MD, Aouji M, Savaiano DA. Yogurt--an autodigesting source of lactose. N Engl J Med. 1984;310:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 209] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | McDonough FE, Hitchins AD, Wong NP, Wells P, Bodwell CE. Modification of sweet acidophilus milk to improve utilization by lactose-intolerant persons. Am J Clin Nutr. 1987;45:570-574. [PubMed] |
| 34. | Gorbach SL. Lactic acid bacteria and human health. Ann Med. 1990;22:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Savaiano DA, AbouElAnouar A, Smith DE, Levitt MD. Lactose malabsorption from yogurt, pasteurized yogurt, sweet acidophilus milk, and cultured milk in lactase-deficient individuals. Am J Clin Nutr. 1984;40:1219-1223. [PubMed] |
| 36. | Mustapha A, Jiang T, Savaiano DA. Improvement of lactose digestion by humans following ingestion of unfermented acidophilus milk: influence of bile sensitivity, lactose transport, and acid tolerance of Lactobacillus acidophilus. J Dairy Sci. 1997;80:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Lin MY, Yen CL, Chen SH. Management of lactose maldigestion by consuming milk containing lactobacilli. Dig Dis Sci. 1998;43:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. Probiotics--compensation for lactase insufficiency. Am J Clin Nutr. 2001;73:421S-429S. [PubMed] |
| 39. | Lerebours E, N'Djitoyap Ndam C, Lavoine A, Hellot MF, Antoine JM, Colin R. Yogurt and fermented-then-pasteurized milk: effects of short-term and long-term ingestion on lactose absorption and mucosal lactase activity in lactase-deficient subjects. Am J Clin Nutr. 1989;49:823-827. [PubMed] |
| 40. | Saltzman JR, Russell RM, Golner B, Barakat S, Dallal GE, Goldin BR. A randomized trial of Lactobacillus acidophilus BG2FO4 to treat lactose intolerance. Am J Clin Nutr. 1999;69:140-146. [PubMed] |
| 41. | Szilagyi A. Prebiotics or probiotics for lactose intolerance: a question of adaptation. Am J Clin Nutr. 1999;70:105-106. [PubMed] |
| 42. | Sherman PM. Probiotics and lactose maldigestion. Can J Gastroenterol. 2004;18:81-82. [PubMed] |
| 43. | Yesovitch R, Cohen A, Szilagyi A. Failure to improve parameters of lactose maldigestion using the multiprobiotic product VSL3 in lactose maldigesters: a pilot study. Can J Gastroenterol. 2004;18:83-86. [PubMed] |
| 44. | Leichter J. Comparison of whole milk and skim milk with aqueous lactose solution in lactose tolerance testing. Am J Clin Nutr. 1973;26:393-396. [PubMed] |
| 45. | Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 307] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Holgate AM, Read NW. Effect of ileal infusion of intralipid on gastrointestinal transit, ileal flow rate, and carbohydrate absorption in humans after ingestion of a liquid meal. Gastroenterology. 1985;88:1005-1011. [PubMed] |
| 47. | Houghton LA, Mangnall YF, Read NW. Effect of incorporating fat into a liquid test meal on the relation between intragastric distribution and gastric emptying in human volunteers. Gut. 1990;31:1226-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Cavalli-Sforza LT, Strata A. Double-blind study on the tolerance of four types of milk in lactose malabsorbers and absorbers. Hum Nutr Clin Nutr. 1987;41:19-30. [PubMed] |
| 49. | Vesa TH, Marteau PR, Briet FB, Boutron-Ruault MC, Rambaud JC. Raising milk energy content retards gastric emptying of lactose in lactose-intolerant humans with little effect on lactose digestion. J Nutr. 1997;127:2316-2320. [PubMed] |
| 50. | Martini MC, Savaiano DA. Reduced intolerance symptoms from lactose consumed during a meal. Am J Clin Nutr. 1988;47:57-60. [PubMed] |
| 51. | Peuhkuri K, Vapaatalo H, Nevala R, Korpela R. Influence of the pharmacological modification of gastric emptying on lactose digestion and gastrointestinal symptoms. Aliment Pharmacol Ther. 1999;13:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Szilagyi A, Salomon R, Seidman E. Influence of loperamide on lactose handling and oral-caecal transit time. Aliment Pharmacol Ther. 1996;10:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Szilagyi A, Torchinsky A, Calacone A. Possible therapeutic use of loperamide for symptoms of lactose intolerance. Can J Gastroenterol. 2000;14:581-587. [PubMed] |
| 54. | Habte D, Sterky G, Hjalmarsson B. Lactose malabsorption in Ethiopian children. Acta Paediatr Scand. 1973;62:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Stephenson LS, Latham MC. Lactose intolerance and milk consumption: the relation of tolerance to symptoms. Am J Clin Nutr. 1974;27:296-303. [PubMed] |
| 56. | Sadre M, Karbasi K. Lactose intolerance in Iran. Am J Clin Nutr. 1979;32:1948-1954. [PubMed] |
| 57. | Johnson AO, Semenya JG, Buchowski MS, Enwonwu CO, Scrimshaw NS. Adaptation of lactose maldigesters to continued milk intakes. Am J Clin Nutr. 1993;58:879-881. [PubMed] |
| 58. | Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64:232-236. [PubMed] |
| 59. | Briet F, Pochart P, Marteau P, Flourie B, Arrigoni E, Rambaud JC. Improved clinical tolerance to chronic lactose ingestion in subjects with lactose intolerance: a placebo effect. Gut. 1997;41:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Hertzler SR, Savaiano DA, Levitt MD. Fecal hydrogen production and consumption measurements. Response to daily lactose ingestion by lactose maldigesters. Dig Dis Sci. 1997;42:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Hill MJ. Bacterial adaptation to lactase deficiency. Delmont, J, ed. Milk intolerances and rejection. Basel, Switzerland: Karger 1983; 22-26. |
| 62. | Perman JA, Modler S, Olson AC. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. Studies in vivo and in vitro. J Clin Invest. 1981;67:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 117] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Haverberg L, Kwon PH, Scrimshaw NS. Comparative tolerance of adolescents of differing ethic backgrounds to lactose-containing and lactose-free dairy drinks. I. Initial experience with a double-blind procedure. Am J Clin Nutr. 1980;33:17-21. [PubMed] |
| 64. | Vonk RJ, Priebe MG, Koetse HA, Stellaard F, Lenoir-Wijnkoop I, Antoine JM, Zhong Y, Huang CY. Lactose intolerance: analysis of underlying factors. Eur J Clin Invest. 2003;33:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |