Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3123
Revised: February 15, 2006
Accepted: February 18, 2006
Published online: May 21, 2006
AIM: To describe the pattern of inheritance and confirm the diagnostic criteria of primary shunt hyperbilirubinaemia (PSH).
METHODS: Forty members of a family pedigree across four generations were included in this study. All family members were interviewed and investigated by physical examination, hematology and liver function test and the pattern of inheritance was analyzed.
RESULTS: Nine of the forty family members suffered primary shunt hyperbilirubinaemia. The mature erythrocytes of the propositus were irregular in shape and size. The pedigree showed transmission of the trait through four generations with equal distribution in male and female. No individual with a primary shunt hyperbilirubinaemia was born to unaffected parents. The penetrance was complete in adult.
CONCLUSION: The pattern of inheritance is autosomal dominant. The abnormality of erythrocytes and decrease in white blood cell could be supplemented in the diagnosis of PSH. The PSH is a genetic disorder and could by renamed as hereditary shunt hyperbilirubinaemia.
- Citation: Wang CL, Liu XW, Lu FG, Wu XP, Ouyang CH, Yang DY. Primary shunt hyperbilirubinaemia in a large four-generation family confirming autosomal dominant genetic disorder. World J Gastroenterol 2006; 12(19): 3123-3125
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3123.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3123
Primary shunt hyperbilirubinaemia (PSH) is a rare clinical jaundice characterized by an elevated unconjugated serum bilirubin, hyperplastic/ineffective erythropoiesis with a normal peripheral red blood cell survival[1]. From the first case described by Israels in 1959, less than 30 PSH cases have been reported in world literature. Most of them were sporadic reports with only 2 small familial analysis[2,3,4]. We present herein a large family pedigree in which the propositus characterized some different from the previous reports and the inheritance pattern was determined to be autosomal dominant, and in which PSH could probably be renamed as hereditary shunt hyperbilirubinaemia.
The propositus is a 28-year-old Chinese man who was admitted to our hospital because of asymptomatic fluctuating jaundice observed over the previous five years. At age 23, the patient first presented with elevated serum unconjugated bilirubin, splenomegaly and anemia. The serum bilirubin fluctuated between 50-90 μmol/L. There was no history of infectious hepatitis, malaria, Schistosomiasis or other significant childhood illness. He denied any alcohol intake and was taking no medications at the time of admission to our hospital and worked in a normal fashion as a farmer.
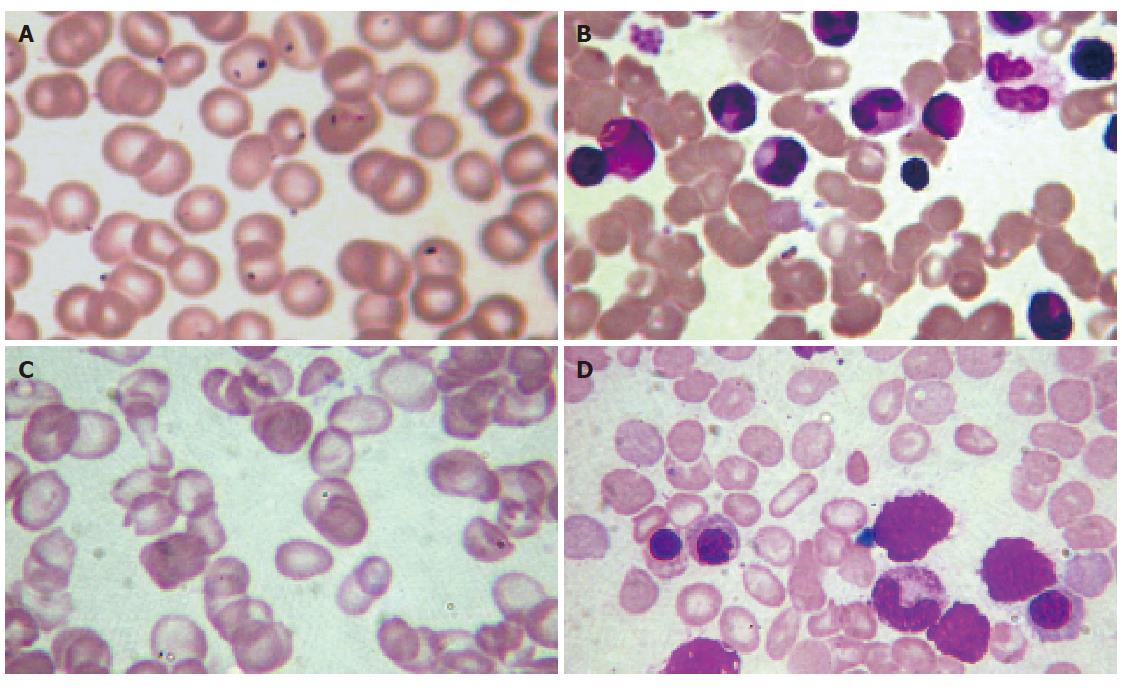
Physical examination revealed a well-developed man of normal stature and growth. Moderate skin icterus was present with mild pallor sclera. The spleen was palpable 4.0 cm below the left costal margin. The liver was impalpable and of normal size on ultrasonography. Murphy’s sign was negative. There was no ascites or peripheral edema, and the neurologic examination was normal (Figure 1A and B).
Initial hematology showed the hemoglobin to be 78 g/L, red blood cell count 3.67×1012/L, MCV 97.5 fl, MCH 34.0 pg, white blood cell count 2.7×109/L and platelets 226×109/L. The reticulocyte count was 3.0%. The differential white cell count was normal. The peripheral smear showed irregular erythrocytes in shape and size with mild macrocytosis and target-, rod-, tear-drop cells (Figure 1C). Total serum protein and albumin were 71.0 g/L and 46.7 g/L, respectively. Alanine aminotransferase (ALT) and serum lactic dehydrogenase (LDH) were normal. Total and conjugated serum bilirubin were 74.2 and 8.8 μmol/L, respectively. Alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT) were normal. Urine urobilinogen was positive. The serum haptoglobin was 0.06 g/L. Serum iron and total iron binding capacity were normal. Glucose-6-phosphate dehydrogenase, pyruvated kinase was normal, as were the Ham’s test and Coombs’ test. The hemoglobin electrophoresis, serum protein electrophoresis, autohemolysis, osmotic fragility (before and after incubation) were all normal. The urine for hemosiderin was negative. Administration of phenobarbital (60 mg/24 h) for one week did not reduce plasma bilirubin to the baseline level (total bilirubin 68.3-64.6 μmol/L).
The bone marrow aspirate revealed a hypercellular marrow due to an erythroid hyperplasia. Of particular interest is in which there were some target-, rod-, tear-drop cells erythrocytes consistent with the peripheral smear (Figure 1D).
The symptoms and signs of the all members through four generations were thoroughly inspected by two gastroenterologists respectively. Four to six milliliter blood samples of each member were collected using potassium citrate as coagulating agent. The peripheral blood cell count and ALT, total serum bilirubin, serum unconjugated bilirubin, serum albumin, blood urea nitrogen, creatinine were detected.
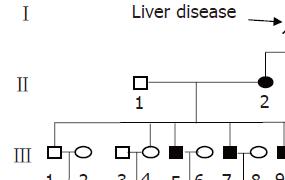
In our patient’s pedigree, the grandfather, mother and maternal aunt showed recessive jaundice (Figure 2). Nine of them suffered from splenomegaly and had elevated unconjugated bilirubin. Among these suffered persons, 5 showed decreased white blood cell counts and 4 showed decreased hemoglobin simultaneously. The patient’s grandfather (I-1), mother (II-2), maternal aunt (II-4), three elder brothers (III-5, III-7, III-9), one cousin (III-14), two nieces (IV-7, IV-15) had clinical features practically identical to that of the propositus. The patient’s immediate family had definitive laboratory corroboration and the same clinical entity distributed equally in male and female.
Shunt hyperbilirubinaemia is a subtype of unconjugated hyperbilirubinaemia and it describes the increase in bilirubin production associated with ineffective erythropoiesis. It has been proposed that ineffective erythropoiesis is related to progenitor inhibition, abnormality on erythropoietin (Epo)/Epo receptor signaling, etc. Secondary shunt hyperbilirubinaemia due to ineffective erythropoiesis can be seen in a number of common clinical disorders, such as thalassemia, myelodysplastic syndrome (MDS) and congenital refractory anemia[5-8]. PSH is a rare disorder with laboratory findings of elevated unconjugated serum bilirubin, mild reticulocytosis, erythroid hyperplasia of bone marrow, splenomegaly and normal peripheral red blood cell survival in the young people[1]. Although some sporadic cases were reported since 1959, the molecular genetic basis has not been previously described because of little pedigree information.
In our case, the clinical findings of the propositus were consistent with the diagnosis of PSH. It is of extreme interest that our patient is unusual in that some abnormal erythrocytes appeared in peripheral smear and bone marrow aspirate, whereas most other reported cases were characterized by normal erythrocytes. It can be proposed that PSH is related to abnormal development of erythrocyte and intramedullary ineffective erythropoiesis. Moreover, the finding of decreased white cell count with normal reticulocyte count indicated the coexistence of splenomegaly and hypersplenism. The abnormal erythrocytes and hypersplenism can be presented as additional clinical findings in PSH diagnosis.
The family pedigree we reported covered four generations. In our patient’s pedigree, the grandfather, mother and maternal aunt showed recessive jaundice. Five of eight (60%) adults in the third generation suffered from this defect. The patient’s immediate family had definitive laboratory corroboration and it appears that they were suffering from the same clinical entity which distributed equally in male and female. It is speculated that the transmission of defect in our patient’s pedigree is an autosomal dominant with equal distribution in male and female. No individual with a PSH was born to unaffected parents. That is, the penetrance was complete in adult. But the severity of clinical features of the disorder was not the same among these affected individuals. Although Bird et al[4] reported that the pattern of inheritance of HSP was X-linked transmission[4], the family pedigree was too small to determine the pattern of inheritance. Here we have provided the first evidence that PSH can be inherited as an autosomal dominant Mendelian trait at least in some cases. Nevertheless, we failed to show male to male transmission of definitely affected individuals which would increase the possibility of chance association. Probably it is associated with the incomplete penetrance in the 4th generation. It remains to be determined whether that refers to all cases of PSH or whether there is a spectrum of different diseases.
Up to now, the precise defect of PSH at the cellular and molecular level remains obscure, although the pathogenesis of PSH centers on markedly ineffective erythropoiesis. Our patient’s pedigree consists of 4 generations, 29 members, and 9 patients. As the largest pedigree of PSH, this family would be suitable for genome-wide scanning to allow linkage or mapping of suitable PSH genes.
We thank all the family pedigree members for the co-operation, especially the propositus Mr. Yue-Neng Wen and Miss Xiao-Yun Qi in collecting the blood samples.
S- Editor Wang J L- Editor Kumar M E- Editor Zhang Y
| 1. | Israels LG, Suderman HJ, Ritzmann SE. Hyperbilirubinaemia due to an alternate pathway of bilirubin production. Am J Med. 1959;27:693. [DOI] [Full Text] |
| 2. | Frank DJ, Dusol M Jr, Schiff ER. Primary shunt hyperbilirubinemia with secondary iron overload: a case report. Gastroenterology. 1979;77:754-757. [PubMed] |
| 3. | Cofrancesco E, Salvatore M, Fenu MP, Pogliani EM. Primary shunt hyperbilirubinemia associated with Gilbert's syndrome. Am J Clin Pathol. 1983;79:627-629. [PubMed] |
| 4. | Bird AR, Knottenbelt E, Jacobs P, Maigrot J. Primary shunt hyperbilirubinaemia: a variant of the congenital dyserythropoietic anaemias. Postgrad Med J. 1991;67:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Cortelezzi A, Cattaneo C, Cristiani S, Duca L, Sarina B, Deliliers GL, Fiorelli G, Cappellini MD. Non-transferrin-bound iron in myelodysplastic syndromes: a marker of ineffective erythropoiesis. Hematol J. 2000;1:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Claessens YE, Bouscary D, Dupont JM, Picard F, Melle J, Gisselbrecht S, Lacombe C, Dreyfus F, Mayeux P, Fontenay-Roupie M. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood. 2002;99:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, Malik P. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol. 2000;28:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Arcasoy MO, Karayal AF, Segal HM, Sinning JG, Forget BG. A novel mutation in the erythropoietin receptor gene is associated with familial erythrocytosis. Blood. 2002;99:3066-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |