Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3096
Revised: December 18, 2005
Accepted: December 22, 2005
Published online: May 21, 2006
AIM: To explore the role of ghrelin in gallstone disease.
METHODS: We carried out a cross-sectional study in 150 subjects, 38 with gallstones (cases) and 112 controls. We also did a real-time PCR-RT study in twenty gallbladder samples each. Body mass index (BMI), serum insulin, ghrelin, and serum lipids were measured. Logistic regression analyses (univariate and multivariate) were conducted to estimate the probability of gallstone disease associated with serum ghrelin concentrations.
RESULTS: Cases were statistically different from controls in gender distribution (P = 0.01), age (53 vs 44 yr, P = 0.002), BMI (28 vs 25; P = 0.004), and glucose (5.26 vs 4.98 mmol/L; P = 0.05). The prevalence of ghrelin serum levels above the third tercile was lower in subjects without metabolic syndrome (P < 0.05). In a multivariate model, we found a protective effect, when ghrelin values were higher than the median value (OR = 0.27, 95%CI 0.09-0.82, P = 0.02). Twenty (20%) gallbladder specimens expressed ghrelin mRNA.
CONCLUSION: Serum ghrelin concentrations are associated with a protective effect of GD.
- Citation: Méndez-Sánchez N, Ponciano-Rodríguez G, Bermejo-Martínez L, Villa AR, Chávez-Tapia NC, Zamora-Valdés D, Pichardo-Bahena R, Barredo-Prieto B, Uribe-Ramos MH, Ramos MH, Baptista-González HA, Uribe M. Low serum levels of ghrelin are associated with gallstone disease. World J Gastroenterol 2006; 12(19): 3096-3100
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3096.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3096
Human obesity is associated with many significant comorbidities, including cardiovascular disease, diabetes mellitus, hypertension, stroke, osteoarthritis, and some cancers, gallstone disease (GD), etc[1]. GD is exceptionally common in Western populations and is a major source of morbidity in the United States[2] and Latin American countries[3]. A relation between obesity and cholesterol gallstones is well recognized. Obese women are more likely to develop gallstones than are obese men[4]. The bile in obese persons is more lithogenic than is that in nonobese persons, ie, the bile of obese persons has a high ratio of cholesterol to solubilizing lipids (bile acids and phospholipids). This high ratio predisposes to crystallization of cholesterol and gallstone formation. The primary reason for lithogenic bile in obese persons is an increase in total body synthesis of cholesterol[5,6].
Ghrelin is an endogenous ligand of the growth hormone secretagogue receptor and a potent stimulator of growth hormone release in humans[7] Ghrelin is synthesized primarily by the stomach, and in substantially lower amounts by the bowel, pituitary, kidney, placenta, and hypothalamus[8]. In both rats and humans, the ghrelin gene is made up of 4 exons and 3 introns and the precursors contain 117 amino acids (preproghrelin). Ghrelin processing results in different splicing and/or posttranslational mechanisms[9]. Two endogenous growth hormones-releasing peptides have been identified, ghrelin and growth hormone-releasing hormone (GHRH) acts on the GHRH receptor to activate adenylate cyclase and increase intracellular cAMP, which serves as a second messenger to activate protein kinase A. This indicates that the GHRH receptor is coupled to a Gs subunit. On the other hand, ghrelin acts on the GHS-R and activates phospholipase C to generate IP3 and diacylglycerol, resulting in an increase of intracellular Ca2+, indicating that the ghrelin receptor is coupled to a Gq subunit[7]
Accumulating evidence suggests that ghrelin contributes to the short- and long-term regulation of body weight as a key element of a complex central signaling network that regulates food intake and energy expenditure[10]; its concentrations increase preprandially and decrease after meals, suggesting a role in meal initiation. Intravenous administration of ghrelin stimulates food intake in humans[11] Ghrelin induces a positive energy balance and induces adiposity in rodents by decreasing fat utilization[12]. Ghrelin concentrations are reported to be decreased in obesity[13] and increased after diet-induced weight loss[14].
The aim of this study was to explore the role of ghrelin in gallstone disease.
We conducted a cross-sectional study, an immuno-histochemistry study and a PCR-RT study in the check-up unit of the Diagnostic Clinic at the Medica Sur Clinic and Foundation (university hospital with subspecialty care) in Mexico City, Mexico. This hospital provides private care for mainly middle- and high-income individuals from Mexico City and the surrounding metropolitan area. Our sample population was formed from a series of consecutive asymptomatic subjects who were referred to the check-up unit by their companies as an annual requirement, not for symptomatic disease. The study included 150 subjects who agreed to participate, 38 subjects found to have GD (25 women, 13 men) and 112 controls (46 women and 66 men without GD). The study was approved by the Human Subjects Committee at The Medica Sur Clinic and Foundation as conforming to the ethical guidelines of the 1975 Declaration of Helsinki, and written informed consent was obtained from all subjects before commencement of the study. All subjects were asked to complete a questionnaire that included demographic and medical variables. The primary demographic and medical variables were gender, age, date of birth, tobacco and alcohol consumption, family history of cardiovascular disease, previous medical conditions, and previous surgery.
Medical exams included an abdominal ultrasonographic study on all subjects as part of the check-up evaluation. Cases were defined as subjects with an ultrasonographic diagnosis of GD (see below), and controls were defined as patients with no such evidence (sensitivity and specificity > 95%)[15].
Abdominal ultrasound was performed using a Sonoline Elegra instrument (Siemens Medical System, Germany) with a 3.5 MHz transducer. Ultrasound diagnosis of GD was assessed by the presence of strong intraluminal echoes that were gravity-dependent or that attenuated ultrasound transmission (acoustic shadowing). All ultrasonographic studies were evaluated, by the same radiologist on two different occasions. Subjects with previous cholecyste-ctomies were not included in the study.
Participants completed a food frequency questionnaire with commonly used portion measures specified. The questionnaire included questions on the frequency and brand of multivitamin and individual vitamin supplements. The answers were subsequently electronically scanned and the daily intake of various nutrients was determined using SNUT software, a program developed by the Instituto Nacional de Salud Pública, Mexico City[16], and appropriate to the Mexican population, in order to estimate dietary energy, protein, carbohydrate, total, saturated, polyunsaturated and monounsaturated fat, vitamin, mineral, and antioxidant intake.
Body weight was measured, in light clothing and without shoes, to the nearest 0.10 kg. Height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Overweight was defined as a BMI ranging from 25 to 29.9 kg/m2 and obesity when BMI was ≥ 30 kg/m2 Waist circumference (to the nearest 0.1 cm) was measured at the midpoint between the lower border of the rib cage and the iliac crest, and hip circumference was similarly obtained at the widest point between hip and buttock.
Insulin concentrations were measured using an immunoenzymometric assay (MEIA; Abbott Diagnostics), with inter- and intra-assay coefficients of variation less than 3%.
Serum glucose in the fasting state was measured in duplicate with an automated analyzer. The coefficient of variation for a single determination was 1.5%. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triacylglycerol concentrations were measured by enzymatic colorimetric methods, using CHOL, HDL-C plus (second generation) and TG assays (Roche Diagnostics Co., Indianapolis, IN, USA), respectively. Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using the Friedewald formula[17].
Assessment of insulin resistance (IR) was made using the Homeostasis Model Assessment (HOMA-IR), as originally described by Matthews et al[18]: HOMA-IR = fasting insulin (U/L) × fasting glucose (mmol/L)/22.5.
Total serum homocysteine was measured with the fully automated Abbott IMx® homocysteine assay (Abbott Laboratories, Abbott Park, IL, USA). Homocysteine is quantified by the intensity of the polarized fluorescent light measured by the IMx optical assembly. Run-to-run CVs (n = 22) were 2.9, 0.8, and 1.7% at total homocysteine concentrations of 7, 12.5, and 25 μmol/L, respectively.
Serum ghrelin concentrations were determined by radioimmunoassay using RIA kits (Linco Research, St. Charles, MO, USA). The intra- and inter-assay coefficients of variation were both less than 5%.
Participants having three or more of the following criteria were defined as having the metabolic syndrome[9]. The waist to hip ratio > 0.85 and high body fat were defined according to previous definitions[19].
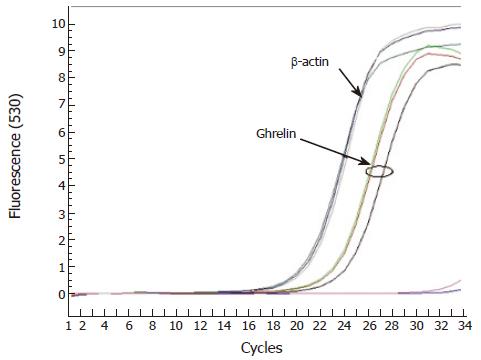
Twenty products of cholecystectomy due to symptomatic gallbladder disease were collected prospectively and frozen at -70 °C until the simple was completed. Then, mRNA was extracted with a one-step technique using guanidine isotiocianate. Complementary DNA was synthesized and processed in real-time PCR using syber green colorant as detector. A qualitative study was performed using β-actin as control.
We established a comparison of continuous variables between cases and controls by means of the non-parametric test U of Mann-Whitney due to the non-normality in some variables. Categorical variables compared between cases and controls were evaluated with Fisher’s exact test (two-tailed). In order to estimate, as main effect, the probability to be a case (GD) according to serum ghrelin values, we built a multivariate model in a logistic regression analysis, adjusted for potential confounders. The association measure derived from this analysis is the odds ratio (OR) estimate of the exponential of the regression coefficient. The statistical analysis was performed using SPSS/PC v12.0 Software (SPSS, Chicago, IL, USA).
Complete information was obtained in 38 cases and 112 controls. We observed differences between cases and controls in some variables, particularly in age (cases were older), in anthropometric variables (cases were more overweight, had central obesity and a higher percentage of body fat), and cases had a tendency to have more insulin resistance and higher serum homocysteine values than controls. The median of serum ghrelin values did not show a statistic difference between cases and controls (Table 1).
| Variable | (median min–max) | ||||
| Cases | Controls | P-value1 | |||
| n = 38 | n = 112 | Mann–Whitney U test | |||
| Age (yr) | 52.5 | 9-80 | 43.5 | 18-76 | 0.002 |
| BMI (kg/m2) | 28.1 | 17-55 | 25 | 19-57 | 0.004 |
| Body fat (%) | 34.1 | 20-45 | 27.9 | 11-47 | < 0.0001 |
| Insulin (U/L) | 6.2 | 2-18 | 5.2 | 2-51 | 0.11 |
| HOMA-IR | 1.4 | 0.4-4.8 | 1.2 | 0.4-14.1 | 0.07 |
| Homocysteine (μmol/L) | 11.0 | 5-25 | 9.0 | 2-20 | 0.007 |
| Total cholesterol (mmol/L) | 4.99 | 2.48-8.28 | 5.07 | 3-8.51 | 0.80 |
| HDL-cholesterol (mmol/L) | 0.98 | 0.59-1.97 | 1.09 | 0.57-2.02 | 0.10 |
| LDL-cholesterol (mmol/L) | 3.1 | 1.47-5.43 | 3.31 | 0.39-6.28 | 0.50 |
| Triacylglycerol (mmol/L) | 1.6 | 0.64-4.54 | 1.42 | 0.46-3.85 | 0.18 |
| Energy (kJ) | 7624 | 4743-11417 | 8323 | 3043-78803 | 0.06 |
| Diet proteins (%) | 16 | 12-24 | 16 | 10-123 | 0.93 |
| Diet lipids (%) | 31 | 22-46 | 34 | 13-500 | 0.05 |
| Diet carbohydrates (%) | 53 | 34-64 | 50 | 13-68 | 0.05 |
| Ghrelin (ng/L) | 660 | 129-1267 | 682 | 117-1467 | 0.71 |
| Physical activity (yes) [n (%)] | 20 (52.6) | 65 (58.0) | 0.581 | ||
| Metabolic syndrome [n (%)] | 15 (39.5) | 18 (16.1) | 0.0061 | ||
In addition, there was a statistic difference in the gender distribution between cases and controls. Cases had more women by proportion than controls (65.8% vs 41.1%, P = 0.01), more hypertension (42.1% vs 11.5%, P < 0.0001) and more metabolic syndrome (39.5% vs 16.1%, P = 0.006) (Table 1).
When analyze the relationship between metabolic syndrome and ghrelin serum levels (in subjects with gallstone disease), we observe that in those subjects with metabolic syndrome (three or more criteria, according ATPIII) the prevalence of ghrelin serum levels above the third tercile was lower than in subjects without metabolic syndrome (26.66% vs 34.78%, P < 0.05) (Table 2) Similar analysis was applied for subjects without GD, but any significant difference was founded (data not shown).
| Ghrelin terciles | Metabolic syndrome criteria | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
| 1st | 0 | 1 | 7 | 4 | 1 | 0 |
| 2nd | 1 | 3 | 3 | 0 | 4 | 2 |
| 3rd | 1 | 5 | 2 | 4 | 0 | 0 |
When we tested the main effect of ghrelin (divided by the median value), a protective association was observed (OR = 0.27, 95%CI 0.09-0.82, P = 0.02), indicating a reduction in the probability of being a case subject if the serum ghrelin values are higher than the median, independent of the effect of the control variables: age (yr), gender (males vs female), diet lipids (%), homocysteine (μmol/L), BMI (kg/m2), HOMA-IR, and physical activity (yes vs no). All the frozen samples showed ghrelin mRNA (Figure 1).
In the present study, we found a statistically significant, negative association of ghrelin serum levels and the prevalence of GD. Obesity is the main risk factor for developing GD and insulin resistance, clinically manifested as the metabolic syndrome. The prevalence of obesity has increased worldwide and is closely associated with an increased morbidity and mortality caused by common diseases of the Western world, such as diabetes, hypertension, coronary heart disease, GD and some cancers[20] Obese individuals are known to have lower ghrelin serum concentrations than healthy controls. In addition, low concentrations of ghrelin are thought to be a consequence of elevated serum levels of insulin and/or leptin, because fasting serum ghrelin concentrations are negatively correlated with fasting serum concentrations of insulin and leptin[13] Recently, ghrelin has been suggested as a regulator of other anorectic cytokines[21] associated with the metabolic syndrome.
Our results could be interpreted as an effect of the negative correlation between ghrelin serum levels and obesity, considering the burden of obesity in GD. However, after controlling for BMI and HOMA-IR, through multivariate analysis, the association remains statistically significant (P < 0.05). Even then, the association could arguably be a product of statistical analysis. However, we believed that there are major facts to be considered.
First, ghrelin was originally described both as a “growth hormone-secretagogue” and a “motilin-related peptide”. Growth hormone has been associated with gallbladder motility, since hypopituitarism is associated with a form of gallbladder dysmotility that resolves after growth hormone replacement[22] and GD is a frequent complication of long-term octreotide treatment in acromegaly[23].
Second, motilin is a well known small intestine-derived factor in the regulation of gastrointestinal motility in humans involved in receptor-induced gastric motility[24] High motilin concentrations can induce growth hormone releasing effects similar to those of ghrelin and high ghrelin concentrations can induce the gastric prokinetic effect of motilin. Luiking et al showed that motilin induces gallbladder emptying in humans[25] and Stolk
et al[26] observed an impaired motilin-response in fasting cholesterol gallstones patients.
Third, obesity-related insulin resistance could be a determinant factor in the pathophysiology of GD. Scragg et al[27] demonstrated higher insulin serum concentration in patients with GD, and an associated increase in the bile cholesterol saturation index[28,29]. In the present study, we observed that cases had lower ghrelin serum levels and higher prevalence of metabolic syndrome, which we have previously reported as a risk factor for GD[30]. Interestingly, Langenberg et al[31] reported that lower ghrelin levels were associated with a significantly higher prevalence of the metabolic syndrome, with progressively lower ghrelin levels as the number of metabolic syndrome components increased.
Fourth, We demonstrated the expression of mRNA of ghrelin in frozen tissue, suggesting that the gallbladder is capable of producing ghrelin.
In conclusion, our results suggest that serum ghrelin concentrations are associated with a protective effect of GD. We speculate that this is related with a motilin-like effect of ghrelin on the gallbladder motility. However, further studies are needed to determine the exact role of ghrelin in gallbladder physiology.
S- Editor Pan BR E- Editor Ma WH
| 1. | Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Méndez-Sánchez N, Jessurun J, Ponciano-Rodríguez G, Alonso-de-Ruiz P, Uribe M, Hernández-Avila M. Prevalence of gallstone disease in Mexico. A necropsy study. Dig Dis Sci. 1993;38:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Méndez-Sánchez N, González V, King-Martínez AC, Sánchez H, Uribe M. Plasma leptin and the cholesterol saturation of bile are correlated in obese women after weight loss. J Nutr. 2002;132:2195-2198. [PubMed] |
| 5. | Bennion LJ, Grundy SM. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975;56:996-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Méndez-Sánchez N, González V, Aguayo P, Sánchez JM, Tanimoto MA, Elizondo J, Uribe M. Fish oil (n-3) polyunsaturated fatty acids beneficially affect biliary cholesterol nucleation time in obese women losing weight. J Nutr. 2001;131:2300-2303. [PubMed] |
| 7. | Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1133] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 8. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 472] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2780] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 13. | Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1328] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 14. | Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1528] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 15. | Calvo MM, Bujanda L, Heras I, Calderon A, Cabriada JL, Orive V, Martinez A, Capelastegi A. Magnetic resonance cholangiography versus ultrasound in the evaluation of the gallbladder. J Clin Gastroenterol. 2002;34:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;40:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 322] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] |
| 18. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24516] [Article Influence: 612.9] [Reference Citation Analysis (1)] |
| 19. | Hattori K, Becque MD, Katch VL, Rocchini AP, Boileau RA, Slaughter MH, Lohman TG. Fat patterning of adolescents. Ann Hum Biol. 1987;14:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Sampliner RE, Bennett PH, Comess LJ, Rose FA, Burch TA. Gallbladder disease in pima indians. Demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970;283:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 230] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57-66. [PubMed] |
| 22. | Moschetta A, Twickler TB, Rehfeld JF, van Ooteghem NA, Cabezas MC, Portincasa P, van Berge-Henegouwen GP, van Erpecum KJ. Effects of growth hormone deficiency and recombinant growth hormone therapy on postprandial gallbladder motility and cholecystokinin release. Dig Dis Sci. 2004;49:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bigg-Wither GW, Ho KK, Grunstein RR, Sullivan CE, Doust BD. Effects of long term octreotide on gall stone formation and gall bladder function. BMJ. 1992;304:1611-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Luiking YC, Peeters TL, Stolk MF, Nieuwenhuijs VB, Portincasa P, Depoortere I, van Berge Henegouwen GP, Akkermans LM. Motilin induces gall bladder emptying and antral contractions in the fasted state in humans. Gut. 1998;42:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Luiking YC, Akkermans LM, van der Reijden AC, Peeters TL, van Berge-Henegouwen GP. Differential effects of motilin on interdigestive motility of the human gastric antrum, pylorus, small intestine and gallbladder. Neurogastroenterol Motil. 2003;15:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Stolk MF, Van Erpecum KJ, Peeters TL, Samsom M, Smout AJ, Akkermans LM, Vanberge-Henegouwen GP. Interdigestive gallbladder emptying, antroduodenal motility, and motilin release patterns are altered in cholesterol gallstone patients. Dig Dis Sci. 2001;46:1328-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Scragg RK, Calvert GD, Oliver JR. Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J (Clin Res Ed). 1984;289:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Nepokroeff CM, Lakshmanan MR, Ness GC, Dugan RE, Porter JW. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974;160:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Osborne AR, Pollock VV, Lagor WR, Ness GC. Identification of insulin-responsive regions in the HMG-CoA reductase promoter. Biochem Biophys Res Commun. 2004;318:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, Sanchez-Lara K, Ponciano-Rodríguez G, Baptista H, Ramos MH, Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653-1657. [PubMed] |
| 31. | Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E. Ghrelin and the metabolic syndrome in older adults. J Clin Endocrinol Metab. 2005;90:6448-6453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |