Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3088
Revised: January 11, 2006
Accepted: January 14, 2006
Published online: May 21, 2006
AIM: To investigate the incidence of bacterial overgrowth in the stomach by using a new endoscopic method in which intragastric hydrogen and methane gases are collected and analyzed.
METHODS: Studies were performed in 490 consecutive patients undergoing esophagogastroscopy. At endoscopy, we intubated the stomach without inflation by air, and 20 mL of intragastric gas was collected through the biopsy channel using a 30 mL syringe. Intragastric hydrogen and methane concentrations were immediately measured by gaschromatography. H pylori infection was also determined by serology.
RESULTS: Most of intragastric hydrogen and methane levels were less than 15 ppm (parts per million). The median hydrogen and methane values (interquartile range) were 3 (1-8) ppm and 2 (1-5) ppm, respectively. The high hydrogen and methane levels for indication of fermentation were decided if the patient had the values more than 90 percentile range in each sample. When a patient had a high level of hydrogen or methane in one or more samples, the patient was considered to have fermentation. The overall incidence of intragastric fermentation was 15.4% (73/473). Intragastric methane levels were higher in the postoperative group than in other groups. None of the mean hydrogen or methane values was related to H pylori infection.
CONCLUSION: Hydrogen and methane gases are more frequently detected in the stomach than expected, regardless of the presence of abdominal symptoms. Previous gastric surgery influences on the growth of methane-producing bacteria in the fasting stomach.
- Citation: Urita Y, Ishihara S, Akimoto T, Kato H, Hara N, Honda Y, Nagai Y, Nakanishi K, Shimada N, Sugimoto M, Miki K. Hydrogen and methane gases are frequently detected in the stomach. World J Gastroenterol 2006; 12(19): 3088-3091
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3088
Hydrogen breath tests have been used to evaluate intestinal transit, bacterial overgrowth, and disaccharidase deficiency[1-8]. As hydrogen production increases when a small amount of carbohydrate is supplied to colonic bacteria, the measurement of breath hydrogen concentration has been proposed as an indicator of carbohydrate malabsorption[2]. Similarly, breath methane excretion, which reflects an indirect measurement of the metabolism of the anaerobic colonic flora, has been measured[9,10]. Methanogenic bacteria utilize hydrogen, carbon dioxide, and then synthesize methane[11]. All methane absorbed from the colon reaches the lung and excretes into the breath[12]. If the fermentation occurs in the stomach, we can detect intragastric hydrogen and/or methane gas. We therefore attempted to collect intragastric gas endoscopically and measure the intragastric hydrogen and methane levels in order to determine the bacterial overgrowth in the stomach.
Studies were performed in 490 consecutive patients (160 men and 315 women, 19-85 years old) undergoing upper endoscopy. None of the patients had a history of use of proton pump inhibitor (PPI), H2-receptor antagonist (H2-RA), antibiotics, steroids, or nonsteroidal anti-inflammatory drugs for a period of at least six month before the investigation. Twelve patients had a previous Billroth I partial gastrectomy.
Endoscopy was performed after a topical anesthesia gargle. At the time of endoscopic examination, we intubated the stomach without inflation by air, and 20 mL of intragastric gas was collected through the biopsy channel using a 30-mL syringe. The first 5 mL was discarded for reduction of dead-space error. Intragastric hydrogen and methane concentrations were immediately measured by gaschromatography using Breath Analyzer TGA-2000 (TERAMECS Co. Ltd. Kyoto) and expressed in parts per million (ppm). Linear accuracy response range was 2 to 150 ppm. After collecting an intragastric gas sample, the endoscopist inflated the stomach by air and observed the gastric mucosa.
At endoscopy, serum H pylori IgG antibody titers were measured with an ELISA method (HM-CAP). A value of >2.2 was considered seropositive and a value of <1.9 was considered seronegative. Patients with a value of neither less than 1.9 nor more than 2.2 were excluded from this study.
Data of intragastric hydrogen and methane were presented as medians, with interquartile ranges because they were not normally distributed. Comparisons of groups were made using the Mann-Whitney U test. A P value less than 0.05 was considered statistically significant.
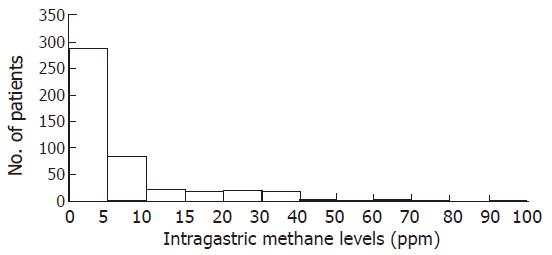
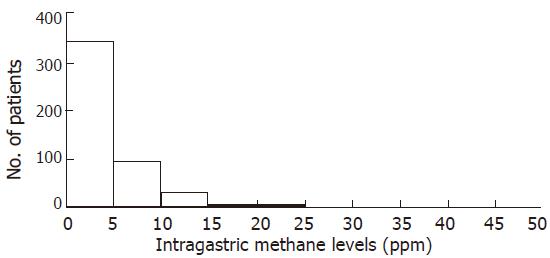
Seventeen patients were dropped from this study because their serum H pylori IgG antibody titers were indeterminate. Figures 1 and 2 show the distribution of intragastric hydrogen and methane levels in the remaining 473 subjects, respectively. Most of the levels were less than 15 ppm. Overall, the median hydrogen and methane values (interquartile range) were 3 (1-8) ppm and 2 (1-5) ppm, respectively. The high hydrogen and methane levels for indication of fermentation were decided if the patient had the values more than 90 percentile range in each sample. Based on this definition, high hydrogen levels were defined as ≥21 ppm and high methane values as ≥8 ppm. In this study, when a patient had a high level of hydrogen or methane in one or more samples, the patient was considered to have fermentation. The overall incidence of intragastric fermentation was 15.4% (73/473). The incidence of intragastric fermentation determined by the intragastric hydrogen level was 11.0% (52/473), whereas those determined by the intragastric methane level was 10.8% (51/473). Of 73 patients with intragastric fermentation, gastric cancer was found in 2 (2/4, 50%), gastric ulcer in 4 (4/38, 11%), duodenal ulcer in 3 (3/15, 20%), previous gastric surgery in 3 (3/12, 25%), and others in 61 patients (61/404, 15%).
Intragastric hydrogen and methane values in relation to endoscopic diagnosis are summarized in Table 1. Intragastric methane levels were significantly higher in the postoperative group than in the gastric ulcer group and in the other groups. Intragastric hydrogen levels were lower in the gastric ulcer group than in other groups but this did not reach statistical significance.
| Gastric ulcer | Duodenal ulcer | Postoperative stomach | Others | |
| No.of patients | 38 | 15 | 12 | 404 |
| H2 (ppm) | 6.8 ± 10.7* | 10.9 ± 17.2 | 11.0 ± 13.9 | 8.2 ± 16.9 |
| P values vs* | * | 0.15 | 0.14 | 0.31 |
| CH4 (ppm) | 3.7 ± 5.6 | 3.7 ± 3.6 | 8.1 ± 12.6** | 3.4 ± 4.1 |
| P values vs** | 0.047 | 0.1 | ** | 0.0002 |
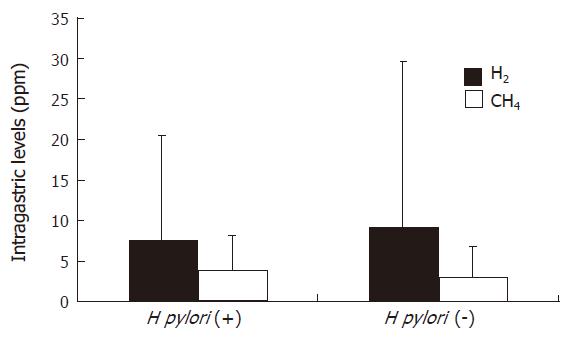
Figure 3 shows the means of intragastric hydrogen and methane concentrations by H pylori status. None of the mean values were related to H pylori infection.
Before the discovery of H pylori infection in 1983[13], it was demonstrated by many investigators that an increased number of bacteria had been found in the stomach in patients with achlorhydria or hypochlorhydria[14]. The type and numbers of microbial flora present in the stomach are affected by gastric pH[15-17], and a rise in intragastric pH has often been associated with an increased number of bacteria in gastric juice[18-20]. Atrophic gastritis is the most common cause of reduced gastric acid secretion, and it often results in bacterial overgrowth[21-23]. It is presently possible to reduce gastric acid secretion with H2-RA or PPI. Treatment with PPI[24-26] or H2-RA[27] induces a clinical state similar to atrophic gastritis with hypochlorhydria and frequently associated with bacterial overgrowth. Recently, there is considerable information on the efficacy of maintenance treatment with reflux esophagitis for up to 1 year[28-30]. As gastric acid plays an important part in the prevention of bacterial colonization of the stomach and the small intestine, reduction of gastric acid secretion by PPI or H2-RA results in gastric and intestinal bacterial overgrowth[24-27,31,32]. In fact, it has been reported a marked increase in bacterial titers in fasting gastric aspirates from patients receiving H2-RA[31,33,34]. On the other hand, the results of identification and quantification of microbes in samples from the gastrointestinal tract are significantly influenced by the culture technique[35].
The most direct method for diagnosing bacterial overgrowth is to perform microbiological cultures after obtaining gastric aspirates. Actually, the microbial flora, which is dominated by Viridans streptococci, coaglase negative Staphylococci, Haemophilus sp.[36], Neisseria spp., Lactobacillus spp., Candida spp., and Aspergillus spp.[31], has been demonstrated. However, the study of gastrointestinal flora by direct methods is cumbersome, primary due to its inaccessible location. In addition, the results of identification and quantification of microbes in samples from the gastrointestinal tract are significantly influenced by difficulties in accurate tube placement, contamination during insertion, delay between sampling and inoculation of culture media, and inadequate anaerobic isolation techniques. In addition, intubation methods are time-consuming, and uncomfortable. Therefore, breath tests were devised as simple alternatives to these invasive tests.
Breath hydrogen measurement is now used in clinical practice to investigate several disorders, including small intestinal disaccharidase deficiencies, intestinal bacterial overgrowth, and orocecal transit time[1-8]. It is based on the ability of the anaerobic microflora of the colon to ferment carbohydrate that has traveled unabsorbed through the small intestine, and to produce hydrogen. This hydrogen is transported to the lungs and exhaled in the expired breath. Although breath tests, such as measuring fasting or postprandial hydrogen concentrations, are a noninvasive method, avoiding the risk of sampling error, it is unable to identify the site of overgrowth. Then, we attempted to measure intragastric hydrogen and methane concentrations so as to determine the site of bacterial overgrowth and the incidence of fermentation in the stomach.
We previously reported the endoscopic 13C-urea breath test (e-UBT)[37] in which intragastric gas was collected and analyzed. Using the same sample collection method as e-UBT, hydrogen and methane gases, which are produced by hydrogen-producing bacteria, could be detected in the stomach. These values were considered to reflect directly the intragastric fermentation.
To the best of our knowledge, our study is the first investigation to measure directly intragastric hydrogen and methane concentrations. The values of intragastric hydrogen concentrations above 21 ppm and methane above 8 ppm were considered abnormal in this study. When a patient had a high level of hydrogen or methane in one or more samples, the patient was considered to have fermentation. The overall incidence of intragastric fermentation was 15.4% (73/473). There was no difference in the incidence of intragastric fermentation determined by hydrogen or methane concentrations. These results reveal that intragastric fermentation is found in more than 15% of patients without medication even after overnight fasting.
In this study, intragastric methane levels were higher in the postoperative group than in other groups, whereas there was a negligible difference of intragastric hydrogen levels between the postoperative stomach group and other groups, thereby suggesting that previous gastric surgery is more closely correlated to methane-producing bacterial overgrowth in the stomach, compared to hydrogen-producing bacterial overgrowth although the exact mechanism is unknown.
On the other hand, Fried et al[26] reported that most of the bacteria identified from the duodenal aspirates belonged to species colonizing the oral cavity and pharynx, suggesting a descending route of colonization. Also, Thompson et al[38] indicated that fermentation of ingested carbohydrate by oropharyngeal bacteria could contribute to measure breath hydrogen values soon after meal ingestion. In addition, many investigators have reported that treatment with PPI[23-25] or H2-RA[26] induces a clinical state similar to atrophic gastritis with hypochlorhydria and a marked increase in bacterial titers in fasting gastric aspirates from patients receiving H2-RA[18,20,21]. Although Husebye et al[36] have reported that fasting hypochlorhydria associated with gastric colonization of microbes belonging to the oro- and nasopharyngeal flora is highly prevalent in healthy old people, atrophic gastritis is the most common cause of reduced gastric acid secretion, and it often results in bacterial overgrowth[21,22]. We previously reported that intestinal metaplasia was detected in 65.4% (358/547) patients with serum IgG antibody to H pylori. Therefore, we expected that a discriminative value might be detected between H pylori-positive and H pylori-negative patients. As shown in Figure 3, none of the mean values were related to H pylori infection.
In summary, we measured directly intragastric hydrogen and methane concentrations using endoscopy and found the incidences of hydrogen and methane production in the stomach. In this new method, the intragastric gas can be easily collected endoscopically and it does not take much time. Hydrogen and methane gases are more frequently detected in the stomach than expected, regardless of the presence of abdominal symptoms. Moreover, previous gastric surgery influences on the production of methane in the fasting stomach and probably the growth of methane-producing bacteria in the upper digestive tract.
S-Editor Wang J L-Editor Kumar M E- Editor Zhang Y
| 1. | Hoekstra JH. Fructose breath hydrogen tests in infants with chronic non-specific diarrhoea. Eur J Pediatr. 1995;154:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Levitt MD, Donaldson RM. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J Lab Clin Med. 1970;75:937-945. [PubMed] |
| 3. | Bond JH Jr, Levitt MD. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972;51:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Urita Y, Hike K, Torii N, Kikuchi Y, Sasajima M, Miki K. Efficacy of lactulose plus 13C-acetate breath test in the diagnosis of gastrointestinal motility disorders. J Gastroenterol. 2002;37:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Bond JH Jr, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546-555. [PubMed] |
| 6. | La Brooy SJ, Male PJ, Beavis AK, Misiewicz JJ. Assessment of the reproducibility of the lactulose H2 breath test as a measure of mouth to caecum transit time. Gut. 1983;24:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Götze H, Ptok A. Orocaecal transit time in patients with Crohn disease. Eur J Pediatr. 1993;152:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | McKay LF, Eastwood MA, Brydon WG. Methane excretion in man--a study of breath, flatus, and faeces. Gut. 1985;26:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Bjørneklett A, Jenssen E. Relationships between hydrogen (H2) and methane (CH4) production in man. Scand J Gastroenterol. 1982;17:985-992. [PubMed] |
| 11. | Stadtman TC. Methane fermentation. Annu Rev Microbiol. 1967;21:121-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 66] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Bond JH Jr, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971;133:572-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 233] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 14. | Garrod LP. A study of the bactericidal power of hydrochloric acid and of gastric juice. St Barth Hosp Rep. 1939;72:145-167. |
| 15. | Gray JD, Shiner M. Influence of gastric pH on gastric and jejunal flora. Gut. 1967;8:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 119] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Drasar BS, Shiner M, McLeod GM. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology. 1969;56:71-79. [PubMed] |
| 17. | Gilman RH, Partanen R, Brown KH, Spira WM, Khanam S, Greenberg B, Bloom SR, Ali A. Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology. 1988;94:1308-1314. [PubMed] |
| 18. | Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 315] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Ruddell WS, Bone ES, Hill MJ, Walters CL. Pathogenesis of gastric cancer in pernicious anaemia. Lancet. 1978;1:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Reed PI, Smith PL, Haines K, House FR, Walters CL. Gastric juice N-nitrosamines in health and gastroduodenal disease. Lancet. 1981;2:550-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Villako K, Tamm A, Savisaar E, Ruttas M. Prevalence of antral and fundic gastritis in a randomly selected group of an Estonian rural population. Scand J Gastroenterol. 1976;11:817-822. [PubMed] |
| 22. | Siurala M, Isokoski M, Varis K, Kekki M. Prevalence of gastritis in a rural population. Bioptic study of subjects selected at random. Scand J Gastroenterol. 1968;3:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 157] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kreuning J, Bosman FT, Kuiper G, Wal AM, Lindeman J. Gastric and duodenal mucosa in 'healthy' individuals. An endoscopic and histopathological study of 50 volunteers. J Clin Pathol. 1978;31:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Sharma BK, Santana IA, Wood EC, Walt RP, Pereira M, Noone P, Smith PL, Walters CL, Pounder RE. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br Med J (Clin Res Ed). 1984;289:717-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Saltzman JR, Kowdley KV, Pedrosa MC, Sepe T, Golner B, Perrone G, Russell RM. Bacterial overgrowth without clinical malabsorption in elderly hypochlorhydric subjects. Gastroenterology. 1994;106:615-623. [PubMed] |
| 26. | Fried M, Siegrist H, Frei R, Froehlich F, Duroux P, Thorens J, Blum A, Bille J, Gonvers JJ, Gyr K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Stockbrugger RW, Cotton PB, Eugenides N, Bartholomew BA, Hill MJ, Walters CL. Intragastric nitrites, nitrosamines, and bacterial overgrowth during cimetidine treatment. Gut. 1982;23:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Klinkenberg-Knol EC, Festen HP, Jansen JB, Lamers CB, Nelis F, Snel P, Lückers A, Dekkers CP, Havu N, Meuwissen SG. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994;121:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 252] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Klinkenberg-Knol EC, Nelis F, Dent J, Snel P, Mitchell B, Prichard P, Lloyd D, Havu N, Frame MH, Romàn J. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 362] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Hallerbäck B, Unge P, Carling L, Edwin B, Glise H, Havu N, Lyrenäs E, Lundberg K. Omeprazole or ranitidine in long-term treatment of reflux esophagitis. The Scandinavian Clinics for United Research Group. Gastroenterology. 1994;107:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Snepar R, Poporad GA, Romano JM, Kobasa WD, Kaye D. Effect of cimetidine and antacid on gastric microbial flora. Infect Immun. 1982;36:518-524. [PubMed] |
| 32. | Ruddell WS, Axon AT, Findlay JM, Bartholomew BA, Hill MJ. Effect of cimetidine on the gastric bacterial flora. Lancet. 1980;1:672-674. [PubMed] |
| 33. | Muscroft TJ, Youngs D, Burdon DW, Keighley MR. Cimetidine and the potential risk of postoperative sepsis. Br J Surg. 1981;68:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Mowat C, Williams C, Gillen D, Hossack M, Gilmour D, Carswell A, Wirz A, Preston T, McColl KE. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology. 2000;119:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Clarke RT, Beauchop T. Methods for studying gut microbes. Microbial ecology of the gut. London: Acaddemic Press 1977; 33. |
| 36. | Husebye E, Skar V, Høverstad T, Melby K. Fasting hypochlorhydria with gram positive gastric flora is highly prevalent in healthy old people. Gut. 1992;33:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Urita Y, Miki K. Endoscopic 13C-urea breath test. Dig Endosc. 2000;12:29-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Thompson DG, O'Brien JD, Hardie JM. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology. 1986;91:853-860. [PubMed] |