Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3055
Revised: January 3, 2006
Accepted: January 9, 2006
Published online: May 21, 2006
AIM: To explore the effect of recombinant human interleukin-11 (rhIL-11) on the expressions of interleukin-11 receptor α-chain (IL-11Rα) and an additional signal transducer glycoprotein 130 (gp130) in intestinal epithelium cell line-6 (IEC-6) after neutron irradiation.
METHODS: Cultured IEC-6 cells were exposed to 4.0Gy neutron and treated with 100 ng/mL rhIL-11 12 h prior to or immediately after irradiation. The apoptosis and necrosis rates and expressions of IL-11Rα and gp130 were observed by flow cytometry, immunohistochemistry, Western blot and image analysis.
RESULTS: The apoptosis rate of IEC-6 cells was increased by irradiation at 6 h (P < 0.01), IL-11 stimulation resulted in a decreased apoptosis rate in irradiated IEC-6 cells (P < 0.05). In normal control IEC-6 cells, intense immunoreactivity of IL-11Rα was located within the cell membrane and cytoplasm. The level of IL-11Rα expression significantly decreased at 6 h after irradiation (P < 0.01) and restored at 24 h after irradiation. In IEC-6 cells treated with both radiation and rhIL-11, the level of IL-11Rα expression was higher than that of irradiated cells (P < 0.05). When it came to gp130 protein, it was located in the cytoplasm of IEC-6 cells. After irradiation, we found a progressive decrease in the expression of gp130 protein (P < 0.05) in 48 h post-radiation, while in rhIL-11-stimulated cells, it came back to normal level at 24 h after irradiation and decreased at 48 h, but was still higher than that of only irradiated cells (P < 0.05).
CONCLUSION: rhIL-11 can protect IEC-6 cells from neutron irradiation. The protective effect of rhIL-11 might be connected with its ability to up-regulate the expressions of specific ligand-binding subunit IL-11Rα and signal-transducing subunit gp130.
- Citation: Wang RJ, Peng RY, Fu KF, Gao YB, Han RG, Hu WH, Luo QL, Ma JJ. Effect of recombinant human interleukin-11 on expressions of interleukin-11 receptor α-chain and glycoprotein 130 in intestinal epithelium cell line-6 after neutron irradiation. World J Gastroenterol 2006; 12(19): 3055-3059
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3055
Intestine is highly sensitive to neutron irradiation. Once exposed to a given neutron irradiation dose, intestine is damaged severely and has become a severe limitation for patients on frequent radiotherapy for abdominal and pelvic malignancies.
Interleukin-11 (IL-11) is a member of a family of cytokines, including interleukin-6 (IL-6), leukemia inhibitory factor, oncostatin M, and ciliary neurotropic factor. IL-11-induced signaling is mediated by the formation of a hexameric receptor complex, composed of two molecules each of IL-11, IL-11Rα, and gp130[1]. Much attention has been paid to its actions on hematopoietic system[2,3]. Recently, IL-11 has been found to reduce intestinal epithelial injury due to a wide variety of causative incidents, including radiation and chemotherapy[4-11]. Yet the mechanisms of its irradiation protection are scarcely reported and no data on the action and mechanisms of IL-11 in the field of neutron irradiation are available.
In the present study, a model of normal rat intestinal epithelium cell line-6 (IEC-6) was established to observe the effect of recombinant interleukin-11 (rhIL-11) on the expressions of interleukin-11 receptor α-chain and gp130 in IEC-6 cells after neutron irradiation, which would be a base for further research of its effects on intestinal damage induced by irradiation and its mechanisms.
IEC-6 cell line was kindly presented by Luo QL (Institute of Radiation Medicine, Academy of Military Medical Sciences, Beijing) and cultured in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% fetal calf serum (FCS), 0.5% ciprofloxacin under standard tissue culture conditions (50 mL/L CO2 at 37 °C). The medium was changed every other day. Before treatment with rhIL-11 (Jiuyuan Genetics Co., Hangzhou), cells were incubated in medium containing 2.5% FCS for 24 h, and subsequently, 100 ng/mL rhIL-11 was administered 12 h prior to or immediately after irradiation. Thus cells in exponential growth period were randomly assigned to four groups: normal control cells, neutron irradiated cells, cells treated with rhIL-11 prior to irradiation (rhIL-11 pretreatment) and cells treated with rhIL-11 after irradiation (rhIL-11 post-treatment).
Culture flasks containing IEC-6 cells were fixed on a tailor-made round plastic plate so that cells could rotate following the plate and absorb rays homogeneously when irradiated. The fission neutron reactor was provided by Institute of Nuclear and New Energy Technology, Tsinghua University. Cells were irradiated at a rate of 39.69cGy/min with a dose of 4.0Gy neutron ray absorbed.
Detection of apoptosis and necrosis was carried out according to the manufacturer’s instructions (Biosea Biotechnology Co., Beijing). IEC-6 cells were detached from culture flasks with 2.5% trypsin (Sigma) to get monocyte suspension with a cell concentration of 5×105-1×106/mL, washed with ice-cold phosphate-buffered saline (PBS), centrifuged twice at 4000 r/min, and resuspended in 200 μL binding buffer. Then 10 μL Annexin-V-FITC and 5 μL propidium iodide (PI) were added for 15 min in the dark, and finally 300 μL binding buffer was added. The apoptosis and necrosis rates of the samples were analyzed by a FACS Calibr flow cytometer (BD Company, American).
IEC-6 cells were seeded onto glass tissue slides. Before staining, slides were fixed in a mixer with methanol and acetone (1:1) for 15 min at 4 °C. After antigen repair with 0.25% trypsin, slides were incubated with rabbit anti- IL-11Rα mAb (1:80, Santa Cruz) for 1h at 37 °C, then at 4 °C overnight, rinsed with PBS and incubated with biotinylated goat anti-rabbit IgG (1:200, Zhongshan Biotechnology Co., Beijing) for 50 min at 37 °C. After rinsed three times with PBS, slides were treated with streptavidin-conjugated peroxidase (1:200, Zhongshan Biotechnology Co., Beijing) for 50 min at 37 °C, incubated for 10-15 min in 3,3’-diaminobenzidine solution (Zhongshan Biotechnology Co., Beijing) at room temperature to allow color development and rinsed with distilled water to quench the reaction. Hematoxylin was used as a counterstain. PBS was used in stead of primary antibody as blank control.
Cells were washed sequentially twice with 1mL ice-cold PBS, centrifuged and resuspended in lysis buffer (1%SDS, 10 mmol/L EDTA, 50 mmol/L Tris-HCL, pH8.1, 1 mmol/L phenylmethylsulfonyl fluoride), sonicated on ice and centrifuged at 12 000 r/min for 15 min at 4 °C. Protein concentration of the supernatant (protein fraction) was determined using a BCA protein assay reagent kit (Pierce). An aliquot of 25 μg of protein was mixed with an equivalent volume of 2× protein loading buffer containing 2-β-mercaptoethanol and boiled for 10 min before it was loaded onto SDS-polyacrylamide gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes and blocked in PBS-T (0.1% Tween 20) containing 10% nonfat dry milk powder. Protein immunoblots were performed using specific antibodies to IL-11 (1:1000, Santa Cruz), IL-11Rα (1:800), gp130 (1:1000, Santa Cruz) and β-actin (1:1000, Santa Cruz). The membranes were further incubated with peroxidase-conjugated secondary antibodies (Zhongshan Biotechnology Co., Beijing), and protein bands were visualized using a commercial chemiluminescence detection kit (ECL Plus, Pierce) as described by the manufacturer. The prestained protein marker was from New England Biolabs Ltd, UK.
The results of immunohistochemistry and Western blot were quantitated using a computer image analysis system (Beijing Aeronautic and Aerospace University) to assess the mean optical density (MOD) and the integral optical density (IOD) of the samples.
Data were expressed as mean ± SD. Results were analyzed by one-way ANOVA using SPSS 11.0 software. P < 0.05 was considered statistically significant.
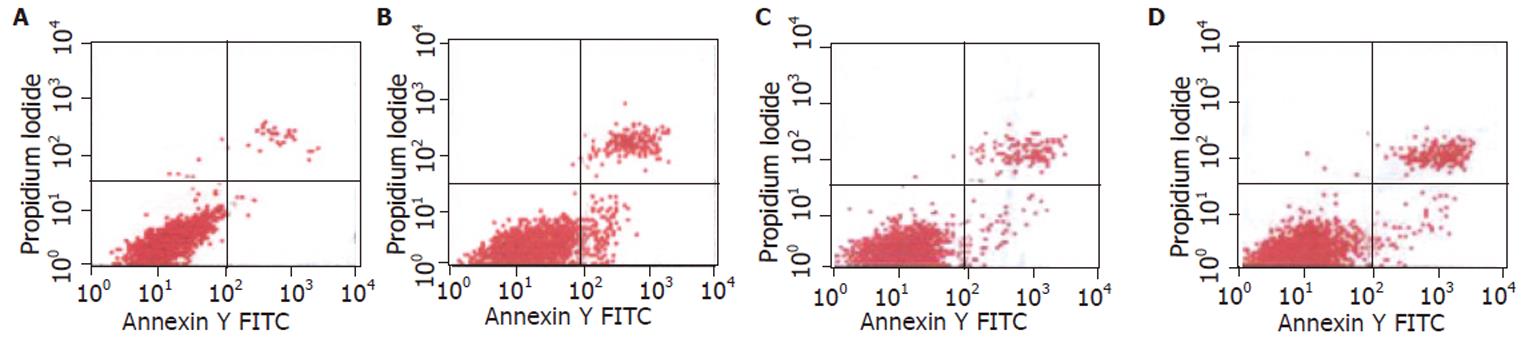
The apoptosis and necrosis rates of IEC-6 cells were increased significantly 6h after 4.0Gy neutron irradiation, while the apoptosis rate of IEC-6 cells treated with rhIL-11 pre- or post-radiation was much lower than that of only irradiated cells, but the necrosis rate remained unchanged (Figure 1).
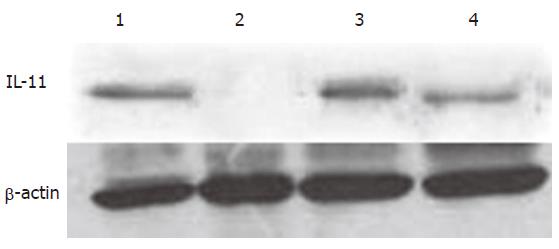
IL-11 expression was found in the cytoplasm of normal IEC-6 cells. The expression of IL-11 was significantly reduced 6 h after 4.0Gy neutron irradiation and returned to normal level 24 h after 4.0Gy neutron irradiation (Figure 2). The quantitative results are shown in Table 1.
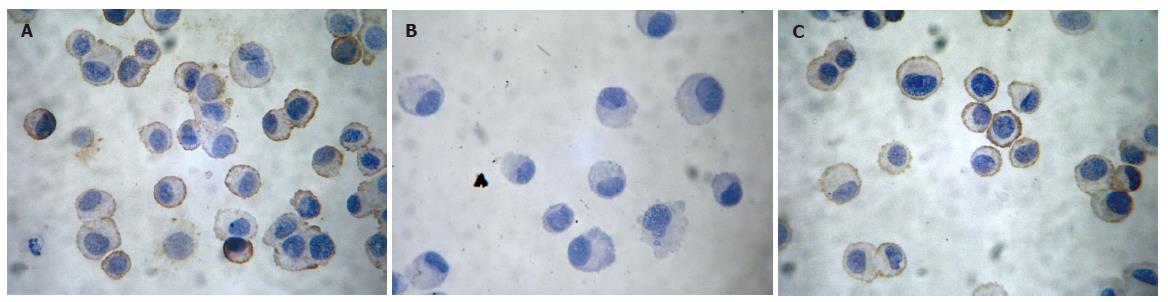
Immunohistochemical staining analysis of IL-11Rαexpression: In normal control IEC-6 cells, intense immunoreactivity of IL-11Rα was located within the cellular membrane and cytoplasm. Six hours after 4.0Gy neutron irradiation, only a very slight immunoreactivity was found but became normal 24 h after irradiation. In IEC-6 cells treated with both radiation and rhIL-11, there was no decrease in the expression of IL-11Rα (Figure 3). The quantitative results are listed in Table 2.
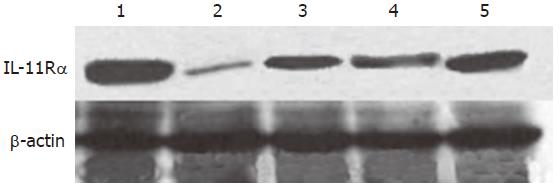
Western blot analysis of IL-11Rαexpression: The results were mainly in accord with those of immunohistochemistry (Figure 4). The quantitative results are listed in Table 3.
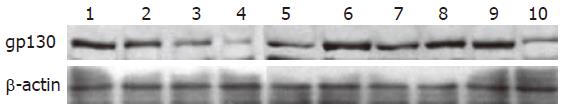
gp130 protein was observed in normal IEC-6 cells. After irradiation, gp130 expression was progressively decreased in within 48 h. In rhIL-11-pretreated cells, despite a decrease 6 h post-irradiation, gp130 expression became normal at 24 h and decreased a little at 48 h. Besides, in IEC-6 cells treated with rhIL-11administration after irradiation, gp expression was decreased slightly 48 h after irradiation and no obvious changes were found 6 and 24 h after irradiation. gp130 protein expression in rhIL-11-stimulated cells 24 h and 48 h after irradiation was higher than that of the irradiated cells (Figure 5). The quantitative results are shown in Table 4.
| Time after irradiation (h) | MOD (×10-1) | IOD (×10-1) | ||||||
| Normal | Neutron-radiated | rhIL-11 pretreatment | rhIL-11 post-treatment | Normal | Neutron- radiated | rhIL-11 pretreatment | rhIL-11 post-reatment | |
| 6 | 4.90 ± 0.44 | 3.70 ± 0.17 | 3.83 ± 0.06 | 5.37 ± 0.58a | 51.07 ± 16.75 | 26.27 ± 10.25b | 35.60 ± 4.03 | 68.60 ± 5.19c |
| 24 | - | 2.70 ± 0.15a | 5.53 ± 0.08c | 5.50 ± 0.11c | - | 19.13 ± 5.14b | 89.79 ± 5.12ad | 75.87 ± 0.68c |
| 48 | - | 1.61 ± 0.10a | 4.47 ± 0.15d | 2.63 ± 0.15c | - | 7.46 ± 0.50b | 41.33 ± 13.55ac | 18.70 ± 0.10bc |
IL-11 is a functionally pleiotropic cytokine originated from the primate PU34 bone marrow fibroblast cell line[12]. Though initially known as a hematopoietic growth factor, IL-11 is an important mediator in systems other than the hematopoietic system. Studies revealed that IL-11 plays an important role in intestinal epithelial injury and repair[4-11], immunomodulation[13-14], neurogenesis[15-16], osteoclastogenesis[17], general homeostasis of the skin, etc[18-19]. Present researches involving intestinal injury and repair on instance intestinal damage induced by chemotherapy and radiation[6,8-11], inflammatory bowel disease[20], bowel ischemical reperfusion injury[4], short bowel syndrome[7], etc, have proved that IL-11 can prevent injury and accelerate recovery.
Previous studies indicated that IL-11 could exert a potent effect on the recovery of the small intestinal mucosa injury in mice treated with radiation and/or chemotherapy by its combined effects on proliferation and apoptosis of crypt cells[9]. However, the effects of IL-11 are not exclusively stimulatory. In vitro studies showed that IL-11 directly interacts with intestinal epithelial cells and reversibly inhibits proliferation of the intestinal crypt stem cell lines (IEC-6 and IEC-18)[21]. It seems contradictory that IL-11 can not only stimulate but inhibit proliferation. Possibly it is because that IL-11 exerts different effects on damaged and undamaged cell clusters. In respect to the undamaged cells, it inhibits proliferation (as reduction of cellular proliferation can be cytoprotective, pretreatment with IL-11 may reduce radiation sensitivity before exposed to rays) and promotes proliferation of damaged cells (enhancing recovery after radiation). Other studies also found that IL-11 pretreatment induces intestinal epithelial cell growth arrest through effects on retinoblastoma protein phosphorylation and delays entry into S phase of the cell cycle[22]. Transient cell cycle arrest is a possible mechanism by which rhIL-11 may protect intestinal epithelial cells from damage induced by chemotherapy or radiation therapy. In this study, pretreatment with rhIL-11 prior to neutron irradiation led to a decrease in apoptosis rate, which may be due to its direct interaction with IEC-6 cells and prolongation of G1-S phase consequently reducing the radiosensitivity of IEC-6 cells. Data also have shown that IL-11 is able to protect intestinal crypt stem cells from impairment of their reproductive capacity by radiation[10]. IL-11-administeration after radiation can cause a greater number of crypt cells to survive a given radiation dose[10]. In this study, a decrease in apoptosis rate was found in cells post-treated with IL-11 after irradiation, which may be due to the fact that rhIL-11 stimulation promotes the survived IEC-6 cells to reproduce, leading to the decrease of apoptosis rate. In addition, 4.0Gy neutron irradiation resulted in a significant reduction of IL-11 expression in IEC-6 cells 6 h after irradiation. Simultaneously, flow cytometry assay showed an increased apoptosis rate, indicating that IL-11 can protect IEC-6 cells from neutron irradiation.
It was reported that IL-11 responsiveness is restricted to cells expressing both the IL-11Rα subunit and the transmembrane signaling receptor gp130 on their surface[23]. The Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway is an important signaling system activated by the IL-11Rα. It was reported that IL-11 stimulation results in decreased activation of caspase-9 and reduced induction of apoptosis in cultured colonic epithelial cells (CEC)[23]. In this study both IL-11Rα and gp130 expressions were decreased in irradiated cells without rhIL-11 treatment 6 h after irradiation (the course of injury). However, in rhIL-11-treated cells, receptor expression level was higher and apoptosis rate was lower than those in irradiated cells without rhIL-11 treatment, indicating that increased IL-11 receptor complex expression induced by rhIL-11 stimulation is involved in the injury and repair of IEC-6 cells caused by neutron irradiation. In irradiated cells, the decreased IL-11 receptor expression is unbeneficial for IL-11 to exert radioprotection effect, thus increasing the apoptosis rate. However in rhIL-11-treated cells, rhIL-11 treatment not only upregulates the expression of IL-11 receptor but also is beneficial to exert its actions, thus reducing the apoptosis rate induced by irradiation. Alterations of receptor expression reflect changes in activation of corresponding signaling pathway. The alteration of IL-11 downstream signal pathway in IEC-6 cells treated with neutron irradiation and rhIL-11 is now under investigation.
The authors thank Dr. Yang Li and Professor Liang-Wen Song for the helpful advices on Western blot and Xiao-Min Wang for the help on image analysis.
S- Editor Wang J L- Editor Wang XL E-Editor Zhang Y
| 1. | Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood. 1997;89:3897-3908. [PubMed] |
| 2. | Nandurkar HH, Robb L, Begley CG. The role of IL-II in hematopoiesis as revealed by a targeted mutation of its receptor. Stem Cells. 1998;16 Suppl 2:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hao J, Luo Q, Xiong G. [The effect of rhIL-11 on hematopoiesis of rhesus monkeys irradiated with 60Co gamma-rays]. Zhonghua Zhong Liu Za Zhi. 2001;23:21-24. [PubMed] |
| 4. | Kuenzler KA, Pearson PY, Schwartz MZ. IL-11 pretreatment reduces cell death after intestinal ischemia-reperfusion. J Surg Res. 2002;108:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG. Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci. 2002;47:2751-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Dickinson EC, Tuncer R, Nadler EP, Koltuksuz U, Boyle P, Alber SM, Watkins SC, Ford HR. Recombinant human interleukin-11 prevents mucosal atrophy and bowel shortening in the defunctionalized intestine. J Pediatr Surg. 2000;35:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Potten CS. Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Orazi A, Du X, Yang Z, Kashai M, Williams DA. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest. 1996;75:33-42. [PubMed] |
| 10. | Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995;62:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Du XX, Doerschuk CM, Orazi A, Williams DA. A bone marrow stromal-derived growth factor, interleukin-11, stimulates recovery of small intestinal mucosal cells after cytoablative therapy. Blood. 1994;83:33-37. [PubMed] |
| 12. | Paul SR, Schendel P. The cloning and biological characterization of recombinant human interleukin 11. Int J Cell Cloning. 1992;10:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Frasca D, Guidi F, Arbitrio M, Pioli C, Leter G, Spano M, Doria G. Use of hematopoietic cytokines to accelerate the recovery of the immune system in irradiated mice. Exp Hematol. 1997;25:1167-1171. [PubMed] |
| 14. | Frasca D, Pioli C, Guidi F, Pucci S, Arbitrio M, Leter G, Doria G. IL-11 synergizes with IL-3 in promoting the recovery of the immune system after irradiation. Int Immunol. 1996;8:1651-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Du X, Everett ET, Wang G, Lee WH, Yang Z, Williams DA. Murine interleukin-11 (IL-11) is expressed at high levels in the hippocampus and expression is developmentally regulated in the testis. J Cell Physiol. 1996;168:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Sims NA, Jenkins BJ, Nakamura A, Quinn JM, Li R, Gillespie MT, Ernst M, Robb L, Martin TJ. Interleukin-11 receptor signaling is required for normal bone remodeling. J Bone Miner Res. 2005;20:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Gyotoku E, Morita E, Kameyoshi Y, Hiragun T, Yamamoto S, Hide M. The IL-6 family cytokines, interleukin-6, interleukin-11, oncostatin M, and leukemia inhibitory factor, enhance mast cell growth through fibroblast-dependent pathway in mice. Arch Dermatol Res. 2001;293:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Scordi IA, Nassiri M, Hanly AJ, Vincek V. Interleukin 11 reduces apoptosis in UVB-irradiated mouse skin. Dermatology. 1999;199:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Greenwood-Van Meerveld B, Venkova K, Keith JC Jr. Recombinant human interleukin-11 restores smooth muscle function in the jejunum and colon of human leukocyte antigen-B27 rats with intestinal inflammation. J Pharmacol Exp Ther. 2001;299:58-66. [PubMed] |
| 21. | Booth C, Potten CS. Effects of IL-11 on the growth of intestinal epithelial cells in vitro. Cell Prolif. 1995;28:581-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Peterson RL, Bozza MM, Dorner AJ. Interleukin-11 induces intestinal epithelial cell growth arrest through effects on retinoblastoma protein phosphorylation. Am J Pathol. 1996;149:895-902. [PubMed] |
| 23. | Kiessling S, Muller-Newen G, Leeb SN, Hausmann M, Rath HC, Strater J, Spottl T, Schlottmann K, Grossmann J, Montero-Julian FA. Functional expression of the interleukin-11 receptor alpha-chain and evidence of antiapoptotic effects in human colonic epithelial cells. J Biol Chem. 2004;279:10304-10315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |