Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3050
Revised: December 2, 2005
Accepted: December 7, 2005
Published online: May 21, 2006
AIM: To develop a method to isolate liver stem cells fast and efficiently.
METHODS: Fetal mouse liver cells were characterized by cell surface antigens (c-Kit and CD45/TER119) using flow cytometry. The candidate liver stem cells were sorted by using immuno-magnetic microbeads and identified by clone-forming culture, RT-PCR and immunofluorescence assays.
RESULTS: The c-Kit–(CD45/TER119)– cell population with 97.9% of purity were purified by immuno-magnetic microbeads at one time. The yield of this separation was about 6% of the total sorting cells and the cell viability was above 98%. When cultured in vitro these cells had high clone-forming and self-renewing ability and expressed markers of hepatocytes and bile duct cells. Functionally mature hepatocytes were observed after 21 d of culture.
CONCLUSION: This method offers an excellent tool for the enrichment of liver stem cells with high purity and viability, which could be used for further studies. It is fast, efficient, simple and not expensive.
- Citation: He YF, Liu YK, Gao DM, Chen J, Yang PY. An efficient method of sorting liver stem cells by using immuno-magnetic microbeads. World J Gastroenterol 2006; 12(19): 3050-3054
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3050
Stem cell is a very small population of cells in various tissues. It is a self-renewing, typically multipotent progenitor with the broadest developmental potential in a particular tissue at a particular time[1,2]. Studies of stem cell have yielded much exciting insight into the field of life science and medicine including cancer, transplantation and proteomics.
Large-scale proteomic study of stem cell, such as two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and mass spectrometry analysis, requires relatively large amounts of protein sample extracted from the cells[3]. For the low-abundance samples such as stem cells, the key to such study relies on the successful obtaining of enough amounts of stem cells with high purity. Two most commonly used methods, magnetic activated cell sorting (MACS) and high-speed fluorescence-activated cell sorting (FACS), have been employed to isolate and purify a special population of cells based on the cell surface markers. In the latter method, however, there are some disadvantages. For example, a high fluid pressure might damage the biological activity of the sorting cells; for rare cells such as stem cells, to isolate large amounts of them is also time-consuming[4].
The liver is the central organ of nutrient digestion and processing, where most of a person’s metabolism occurs. Although liver regeneration is not dependent on a small group of progenitor or stem cells, which may appear in large numbers when mature hepatocytes are inhibited from proliferation[5], it is believed that in a growing liver both hepatocytes and cholangiocytes differentiate from a common progenitor, or liver stem cell[6]. To date, few liver stem cell-specific markers are available, and some are also shared by hematopoietic progenitors[7,8]. Recently, by using FACS liver stem cells have been isolated based on some non-specific cell surface markers (c-Kit, CD45, TER119, c-Met and Dlk) and identified[6,9-11]. In the study reported here, using immuno-magnetic microbeads, we sorted large amounts of candidate liver stem cells with high purity and identified them with self-renewing capability and multilineage differentiation potential.
Animal experiments involving mice were approved by the Institute’s Animal Care and Use Committee. BALB/c mice were maintained under pathogen-free conditions. Day 0 of gestation was taken to be detection of a vaginal plug after overnight mating. Embryos were dissected and fetal liver cell suspensions were prepared by mechanical dissociation and then passed through a 30 μm nylon mesh to remove clumps.
Dissociated liver cells were incubated at 4 °C for 10 min with biotinylated anti-CD45.2, TER119 monoclonal antibodies (mAbs) (eBioscience, San Diego, CA). After three washes with staining medium (PBS containing 3% FBS), cells were incubated with FITC-conjugated anti-c-Kit mAb (eBioscience), and streptavidin-labeled phycoerythrin (eBioscience) at 4 °C for 10 min. Finally, cells were washed three times and resuspended in staining medium. The labeled cells were analyzed and sorted with FACSAria (Becton Dickinson). The acquisition rate was set at 35 000 events per second. Gating was implemented based on negative control staining profiles.
Dissociated fetal liver cells were first stained with biotinylated anti-CD45.2, TER119 and/or c-Kit mAbs at 4 °C for 10 min. Cells were washed twice with PBS containing 0.5% BSA and 2 mmol/L EDTA. Enrichment of target cells by magnetic microbeads was carried out according to the manufacturer’s (Milteny Biotec, Bergisch Gladbach, Germany) protocol. Briefly, cells were resuspended in 80 μL of the same buffer per 107 total cells and incubated with anti-biotin microbeads for 15 min at 6-12 °C. Cells were washed twice and finally resuspended in 500 μL of buffer per 107 total cells. A pre-moistened MS column was placed in the magnetic field of a suitable MACS separator. The cell suspension was applied onto the column and then washed three times. Effluents were collected as the negative fraction. Magnetically labeled cells as the positive fraction were collected by flushing the column with buffer after the column was placed outside of the magnetic field. The sorted cells were then incubated at 4 °C for 10 min with phycoerythrin-streptavidin and analyzed with FACSCalibur (Becton Dickinson).
Cell culture was performed as described previously with minor modifications[9,10]. Liver cell populations were plated onto 20 μg/mL laminin-coated (Gibco BRL, Gaithersburg, MD) 24-well plates or chamber slides at a density of 1000 cells/cm2 and maintained at 37 °C, with 50 mL/L CO2 for 8-21 d. The medium was DMEM/F12 supplemented with 10% FCS, 2 mmol/L L-glutamine, 50 μmol/L 2-mercaptoethanol, 100 U/mL penicillin-streptomycin, 1 × nonessential amino acids (all from Gibco BRL), 1 μg/mL
human insulin and 10–7 mol/L dexamethasone (Sigma-Aldrich, St. Louis, MO) renewed every 4 d. Mouse recombinant hepatocyte growth factor (40 ng/mL) and OSM (10 ng/mL) (R&D system, Minneapolis, MN) were added 24 h after culture initiation.
Detection of hepatocyte or cholangiocyte marker gene expression by RT-PCR was conducted as described[12]. After treated with DNase I (Promega, Madison, WI), equal amounts of RNAs were reverse-transcribed and the cDNAs were amplified by the PCR with gene-specific primers at 94 °C for denaturing, 54 °C for annealing, and 72 °C for extension. β-actin mRNA was used as an internal control. Previous experiments determined 28 cycles to be optimal. The gene-specific intron-spanning primers used are as follows: α-fetoprotein (AFP,amplification of 821 bp), sense 5’-TCACACCCGCTTCCCTCATCCT-3’, antisense 5’-CATCCTGCAGACACTCCAG-3’; albumin (ALB, amplification of 705 bp), sense 5’-AGAAGACACCCTGATTACTCT-3’, antisense 5’-TCGAGAAGCAGGTGTCCTTGT-3’; cytokeratin 19 (CK19,amplification of 566 bp), sense 5’-GTCCTACAGATTGACAATGCTCG-3’, antisense 5’-CTGATCACGCTCTGGATCTGTG-3’; β-actin, (amplification of 390 bp), sense 5’-GTCATCACTATTGGCAACGAGCG-3’, antisense 5’-CTAGAAGCACTTGCGGTGCACG-3’. The amplified products were analyzed by electrophoresis on 1% agarose gels and stained with ethidium bromide.
Cultured cells were washed with PBS and fixed and permeabilized with methanol/acetone (95:5) for 20 min at –20 °C. They were then incubated with the primary Ab goat anti-mouse ALB (Bethyl,Montgomery, TX) and mouse anti-CK19 (DakoCytomation,Denmark) in a moist chamber for 16 h at 4 °C. After washing in PBS, cells were incubated with Cy3-conjugated rabbit anti-goat IgG (Sigma) and FITC-conjugated bovine anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) for 3 h at 4 °C. Nuclei were counterstained with 300 nmol/L DAPI in PBS for 5 min. After final washing, cells were observed using a laser scanning microscope.
Cultured cells were fixed in 4% phosphate-buffered paraformaldehyde and then oxidized in 1% periodic acid for 5 min, rinsed in dH2O three times, treated with Schiff’s reagent for 15 min, rinsed again in dH2O, stained with Mayer’s hematoxylin for 1 min, and finally rinsed in dH2O.
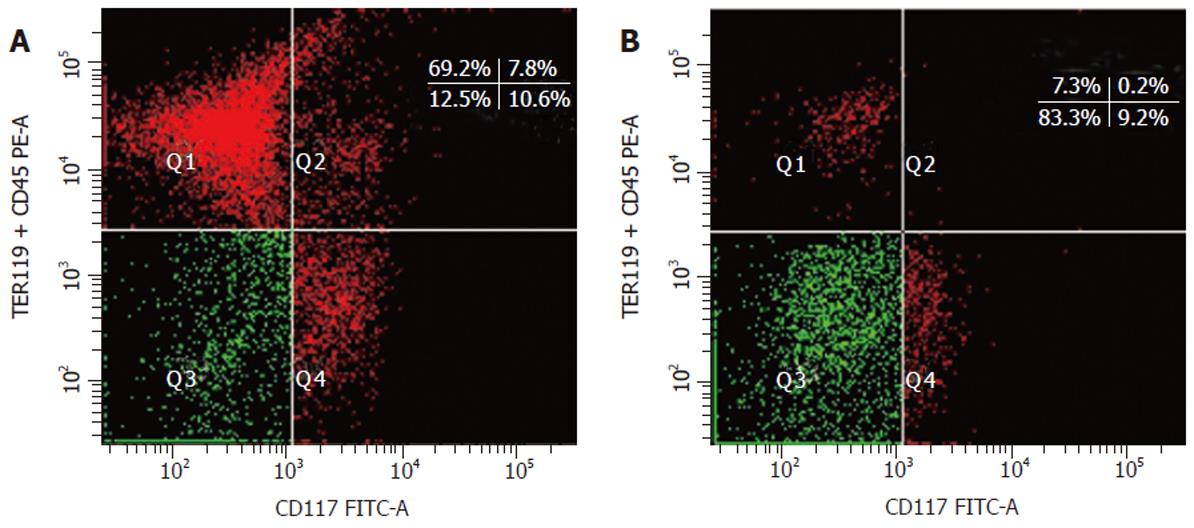
Embryo fetal liver contains lymphohematopoietic progenitors characterized by the CD45 and TER119 antigens. Double staining of embryonic day (ED) 12 fetal mouse liver cells with anti-(TER119 + CD45) mAb and anti-c-Kit (also CD117) mAb revealed four cell populations (Figure 1A). The Q3 c-Kit–(CD45/TER119)– cell population, which had no obvious boundary but continuous fluorescence distribution with the Q4 cell population, was considered to be liver stem cell or contain liver stem cell[9,10]. Flow cytometric sorting for Q3 cells revealed the purity of the sorted cells was 83.3% (Figure 1B). We observed that most of the non-c-Kit–(CD45/TER119)– cells were from Q4 cells near Q3 cell population.
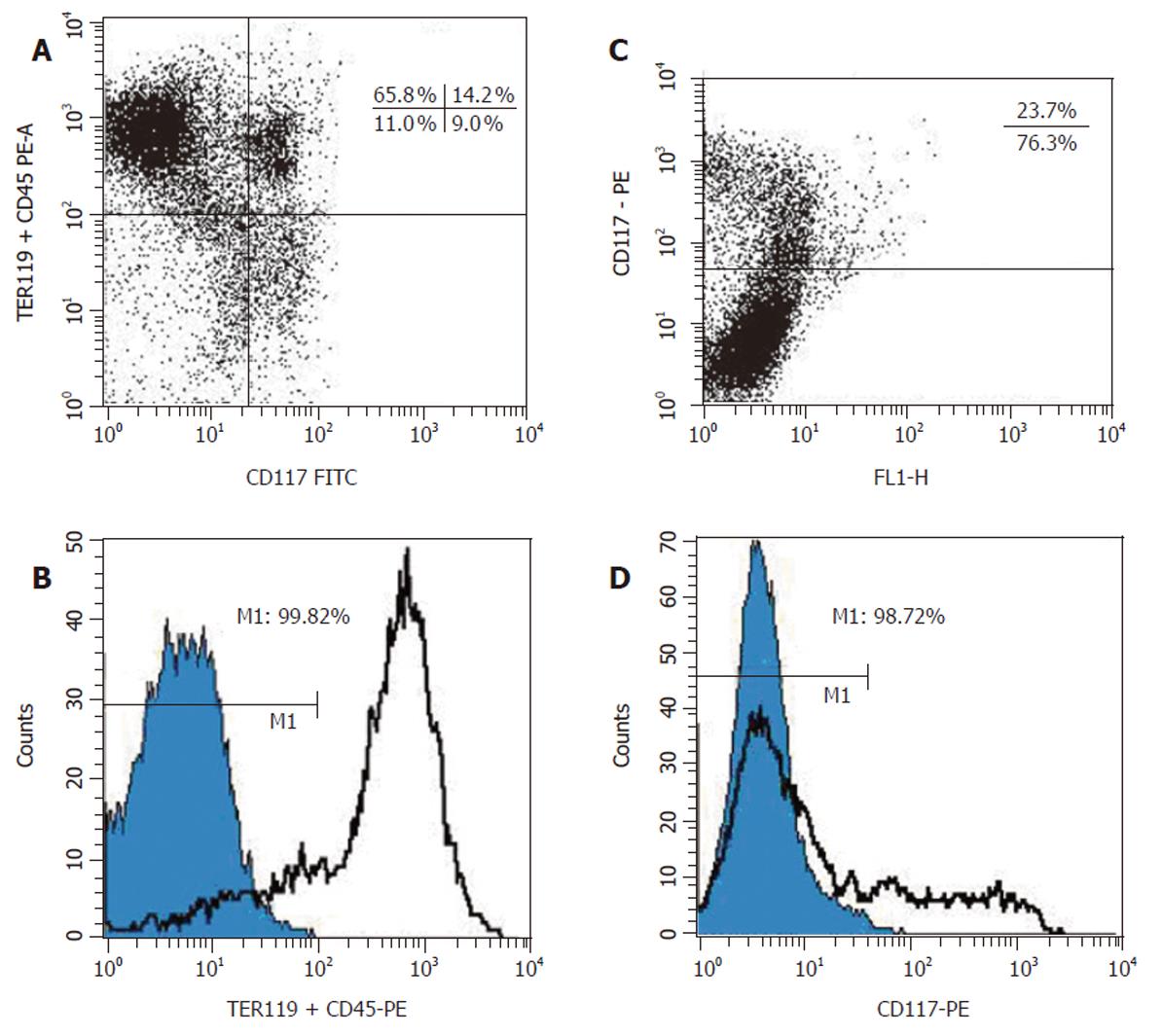
ED 13 fetal mouse liver cells were analyzed in FACSCalibur (Figure 2A) and similar result was obtained to that shown in Figure 1A. The fraction of (CD45/TER119)– and c-Kit– cell population was 20% and 76.8%, respectively. Immuno-magnetic microbeads were used to separate these two cell populations. After double labeled with the biotinylated primary anti-mAb TER119 and CD45.2, which was then recognized by a monoclonal anti-biotin antibody coupled to microbeads, total fetal liver cells were applied onto the separation column in a magnetic field and only the unlabeled cells (negative fraction, both TER119– and CD45–) were passed through. FACS analysis of the negative fraction revealed that more than 99% of the cells were (TER119/CD45.2)– (Figure 2B), indicating that the purity of the sorted cells was above 99%. To separate c-Kit– cell population from the liver cells, a biotinylated anti-c-Kit mAb was used. Total fetal liver cells stained with this mAb were first analyzed by FACS (Figure 2C) and the result was similar to that from FITC-labeled anti-c-Kit mAb (Figure 1A). Similar procedure using anti-biotin antibody coupled microbeads as described above was performed to sort c-Kit– cell population. Figure 2D shows the FACS analytical result of the negative fraction and it was revealed that more than 98% of the cells were c-Kit– cells.
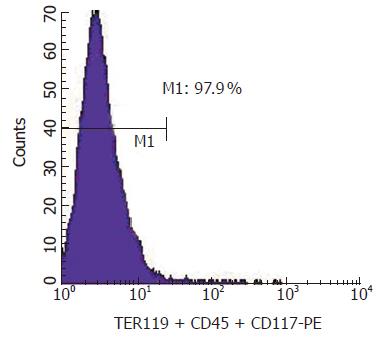
Then we wondered whether these two separation procedures could be combined together and performed once to obtain the c-Kit–(CD45/TER119)– cell population. Theoretically any unwanted cell that expressed either c-Kit or CD45/TER119 could be magnetically labeled and retained as a positive fraction on the separation column in a magnetic field and only the unlabeled cells, the c-Kit–(CD45/TER119)– cells, could pass through. Our experiments indicated that this is feasible. After labeled with a cocktail of biotinylated antibodies (c-Kit, TER119 and CD45.2) and recognized by the anti-biotin microbeads, fetal liver cells were applied onto the separation column and the negative fraction was obtained. The purity of the c-Kit–(CD45/TER119)– cells was 97.9% (Figure 3). The yield of this separation was about 6% of the total sorting cells. The cell viability was above 98% as measured by trypan blue dye exclusion.
To identify whether the immuno-magnetically separated c-Kit–(CD45/TER119)– cells were liver stem cells, their capacity of colony-forming and multilineage differentiation was tested. After cultured in vitro for 4 d, obvious colonies (> 50 cells) were generated. Sorting for c-Kit–(CD45/TER119)– cells (negative fraction) achieved more than 15-fold enrichment of colony-forming cells compared with total fetal liver cells, while the positive fraction of liver cell populations did not generate any colony (only three clones in 96 wells, probably due to c-Kit–(CD45/TER119)– cell contaminants; data not shown). When we replated the cells from the formed clone onto new culture plates, several of these subcultured cells again formed colonies in 5 d (Figure 4).
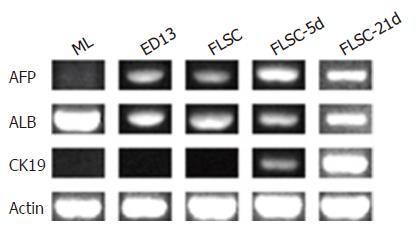
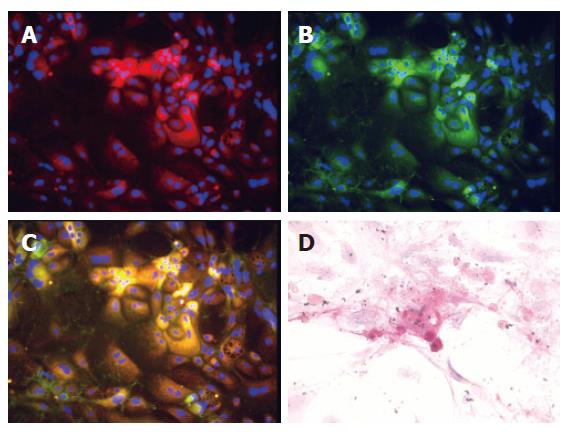
The hepatic differentiation of the cultured cells at d 5 and 21 was tested by their gene expression in RT-PCRs (afp, alb and ck19 genes, Figure 5). AFP was expressed throughout the culture process. The expression of lineage markers ALB (for hepatocyte) and CK19 (for cholangiocyte) was also detected. To verify the RT-PCR results, we stained colony-constituent cells at d 12 with antibodies against albumin or cytokeratin 19. As shown in Figure 6, immunofluorescence revealed that most of cells expressed either albumin or cytokeratin 19 (Figures 6A and B). Two-color ALB/CK19 analysis revealed that ALB+CK19+ cells frequently accumulated in clusters, with hepatocytes and bile duct cells in peripheral segregated areas (Figure 6C). Periodic acid-Schiff staining showed that some cells had abundant glycogen stores at d 21 (Figure 6D), indicating functionally mature hepatocytes were generated. Taken together, these results suggest that the sorted c-Kit–(CD45/TER119)– cells were capable of colony-forming and self-renewing and multilineage differentiation.
Separation of stem cells is typically done based on surface-marker expression. Previous studies showed that liver stem cells were enriched in the c-Kit–CD49f+CD29+CD45–TER119– fraction of mouse fetal liver cells by using flow cytometry and single cell-based assays[9] and were further enriched in the c-Met+CD49f+/lowc-Kit–CD45–TER119– fraction[6]. Whereas oval cells, found in adult liver and marked for c-Kit+CD34+Thy-1+, have been considered to be close descendants of these stem cells[6]. Similarly, about 10% of total ED 11 mouse fetal liver cells, marked for c-Kitlow(CD45/TER119)–, showed in another study, was purified by FACS and considered to contain the earliest-described hepatic progenitors, also displaying features of liver stem cells[10]. Furthermore, a population of hepatoblasts in ED 14.5 fetal liver was isolated based on the expression of Dlk/Pref-1, a type I membrane protein. However, such Dlk+ hepatoblasts represent more mature cells than c-Met+CD49f+/lowc-Kit–CD45–TER119– cells[11]. Taken together the results of these studies, we concluded that liver stem cells could be enriched in the c-Kit–CD45–TER119– population. Our results presented in this report indicate that the c-Kit–CD45–TER119– population, purified by immuno-magnetic microbeads from ED 13 mouse fetal liver, was liver stem cells or enriched the liver stem cells significantly. As the markers for this population (c-kit and TER119/CD45) are all negative, it is easy to use magnetic microbeads to separate these cells at once.
As c-Met was considered to be the positive marker for liver stem cells, as described previously[6], it is also feasible to further separate the c-Met+ population from the sorted c-Kit–(CD45/TER119)– cells by positive selection using immuno-magnetic microbeads. Also since the liver stem cells have been pre-enriched in c-Kit–(CD45/TER119)–, using FACS to separate c-Met+ cells could also be efficient.
There are several advantages of sorting liver stem cells using immuno-magnetic microbeads. First, this method is simple and inexpensive. Second, using this method, we can sort a total of 5 × 107 labeled fetal liver cells and obtain target cells with a high purity in 5 min, as compared with about 5 h by using an FACSAria cell sorter. As for proteomic analysis such as 2D-PAGE, 106-107 of cells with a high purity are needed, according to the dimension of the gel and staining method. Third, magnetically enriched populations help to reduce the prolonged exposure of liver stem cells to the high-pressure fluid environment in the flow cytometry, which might damage the biological activity of such a sensitive cell population. For biochemical and functional studies of stem cells, the total process relies on the viability of isolated cells which requires special attention during the cell isolation and purification procedures. In our experiments, the viability of the sorted liver stem cells by FACS was usually about 85%, as compared to above 98% by using immuno-magnetic microbeads. Fast and efficient acquisition of a large number of liver stem cells with high purity and viability is the basis for proteomic studies of liver stem cells.
In conclusion, the present study shows the application of immuno-magnetic microbeads for sorting and enrichment of liver stem cells from mouse fetal liver, by which the biological activity of the cells is kept. This method is fast, efficient, simple and not expensive.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6917] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 2. | Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1109] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 3. | Unwin RD, Gaskell SJ, Evans CA, Whetton AD. The potential for proteomic definition of stem cell populations. Exp Hematol. 2003;31:1147-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Gangopadhyay NN, Shen H, Landreneau R, Luketich JD, Schuchert MJ. Isolation and tracking of a rare lymphoid progenitor cell which facilitates bone marrow transplantation in mice. J Immunol Methods. 2004;292:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2468] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 6. | Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511-516. [PubMed] |
| 8. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 9. | Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Minguet S, Cortegano I, Gonzalo P, Martínez-Marin JA, de Andrés B, Salas C, Melero D, Gaspar ML, Marcos MA. A population of c-Kit(low)(CD45/TER119)- hepatic cell progenitors of 11-day postcoitus mouse embryo liver reconstitutes cell-depleted liver organoids. J Clin Invest. 2003;112:1152-1163. [PubMed] |
| 11. | Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |