Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3031
Revised: October 15, 2005
Accepted: October 26, 2005
Published online: May 21, 2006
AIM: Lafutidine, a histamine H2 receptor antagonist, exhibits gastro-protective action mediated by capsaicin-sensitive afferent neurons (CSN). We compared the effect between lafutidine and capsaicin, with respect to the interaction with endogenous prostaglandins (PG), nitric oxide (NO) and the afferent neurons, including transient receptor potential vanilloid subtype 1 (TRPV1).
METHODS: Male SD rats and C57BL/6 mice, both wild-type and prostacyclin IP receptor knockout animals, were used after 18 h of fasting. Gastric lesions were induced by the po administration of HCl/ethanol (60% in 150 mmol/L HCl) in a volume of 1 mL for rats or 0.3 mL for mice.
RESULTS: Both lafutidine and capsaicin (1-10 mg/kg, po) afforded dose-dependent protection against HCl/ethanol in rats and mice. The effects were attenuated by both the ablation of CSN and pretreatment with NG-nitro-L-arginine methyl ester, yet only the effect of capsaicin was mitigated by prior administration of capsazepine, the TRPV1 antagonist, as well as indomethacin. Lafutidine protected the stomach against HCl/ethanol in IP receptor knockout mice, similar to wild-type animals, while capsaicin failed to afford protection in the animals lacking IP receptors. Neither of these agents affected the mucosal PGE2 or 6-keto PGF1α contents in rat stomachs. Capsaicin evoked an increase in [Ca2+]i in rat TRPV1-transfected HEK293 cells while lafutidine did not.
CONCLUSION: These results suggest that although both lafutidine and capsaicin exhibit gastro-protective action mediated by CSN, the mode of their effects differs regarding the dependency on endogenous PGs/IP receptors and TRPV1. It is assumed that lafutidine interacts with CSN at yet unidentified sites other than TRPV1.
- Citation: Fukushima K, Aoi Y, Kato S, Takeuchi K. Gastro-protective action of lafutidine mediated by capsaicin-sensitive afferent neurons without interaction with TRPV1 and involvement of endogenous prostaglandins. World J Gastroenterol 2006; 12(19): 3031-3037
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3031.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3031
The mucosal integrity of the stomach is maintained by multiple factors, both paracrine and neuronal systems[1-4]. Prostaglandins (PGs)[1] and nitric oxide (NO)[4] are part of the paracrine system while capsaicin-sensitive afferent neurons play a central role in the neuronal protection of the stomach[2,3]. Studies have demonstrated that capsaicin, a selective stimulator of these afferent neurons, protects the gastric mucosa against various ulcerogenic stimuli such as necrotizing agents[2]. The protective action of capsaicin is mediated by these afferent neurons, because it is totally attenuated by chemical ablation of the neurons following pretreatment with a large dose of capsaicin[2,5]. Recently, the binding site of capsaicin has been identified and named transient receptor potential vaniloid subtype 1 (TRPV1)[6-8]. It is thought that capsaicin stimulates these afferent neurons through the activation of TRPV1, resulting in the release of the neurotransmitter calcitonin gene-related peptide (CGRP) and gastric protection[3].
Previous studies suggest that endogenous PGs sensitize sensory neurons to nociceptive stimulation[9,10]. Boku et al[11] recently reported a lack of release of CGRP in response to mild injury in the stomach of IP-receptor knockout mice. Ohishi et al[9] demonstrated using IP-receptor knockout mice that PGI2 is a major nociceptive mediator in the acetic acid-induced writhing reaction. Since capsaicin-induced gastric cytoprotection was attenuated by indomethacin and disappeared in IP-receptor knockout mice, it is assumed that endogenous prostacyclin (PGI2) plays a supportive role in the mechanism of capsaicin-induced gastric cytoprotection, probably by sensitizing the sensory neurons through IP receptors[12].
Lafutidine, a histamine H2-receptor antagonist [(±)-2-(furfurylsulfinyl)-N-[4-[4-(piperidinomethyl)-2-pyridyl]oxy-(Z)-2-butenyl]acetamide], is known to exhibit potent protective activity in the gastrointestinal mucosa, in addition to gastric anti-secretory action[13-15]. The protective action of lafutidine is independent of the anti-secretory effect and mediated by capsaicin-sensitive sensory neurons[14,15]. Indeed, the protective action of lafutidine is also abrogated by pretreatment with the CGRP antagonist CGRP8-37 and the inhibitor of NO production NG-nitro-L-arginine methyl ester (L-NAME), in addition to chemical ablation of capsaicin-sensitive afferent neurons[16]. However, it remains unknown whether this action of lafutidine results from interaction with TRPV1 or whether endogenous PGs are essential to the gastro-protective effect.
In the present study, we investigated the protective action of lafutidine with respect to the dependence on endogenous PGs and the interaction with TRPV1, and compared the results with those for capsaicin. In addition, we also examined the effect of lafutidine on HCl/ethanol-induced gastric lesions in mice lacking IP receptors, in an attempt to confirm the distinctive mechanism underlying the gastro-protection provided by lafutidine and capsaicin.
Male Sprague-Dawley rats (200-220 g; Nippon Charles River, Shizuoka, Japan) and C57BL/6 mice (25-30 g) were used. Mice lacking prostacyclin (PGI2) IP-receptors were generated as described previously[11]. In brief, each gene encoding the IP receptors was individually disrupted, and chimeric mice were generated. These animals were then back crossed with C57BL/6 mice, and the resulting heterozygous litter mates [IP (+/-),] were bred to produce homozygous IP (-/-) mice. Homozygous mice were born at the predicted Mendelian frequency, grew normally, lived longer than 1 year and were fertile. The distribution of the IP receptor genes was verified by northern blot hybridization, which failed to detect messenger RNA encoding the respective receptors in IP (-/-) mice. The animals were deprived of food but allowed free access to tap water for 18 hr before the experiments. All studies were performed using 4-6 animals per group under unanesthetized conditions. The experimental procedures described here were approved by the Experimental Animal Research Committee of Kyoto Pharmaceutical University.
The rats were given 1 mL of HCl/ethanol (60% in 150 mmo/L HCl) po through esophageal catheter, and killed 1 h later under deep ether anesthesia. The stomach was removed, inflated by injecting 10 mL of 1% formalin for 10 min to fix the inner wall, and opened along the greater curvature. The area (mm2) of hemorrhagic lesions developed in the stomach was measured under a dissecting microscope with a square grid (×10). Lafutidine (1-10 mg/kg) or capsaicin (1-10 mg/kg) was given po 30 min before the administration of HCl/ethanol. In some cases, indomethacin (5 mg/kg, sc), L-NAME (20 mg/kg, sc) or capsazepine (TRPV1 antagonist: 12.5 mg/kg, po) was given 1 h or 30 min, respectively, before the administration of lafutidine or capsaicin. In addition, the protective effect of lafutidine and capsaicin against HCl/ethanol was also examined in rats with ablation of capsaicin-sensitive sensory neurons (chemical deafferentation). Chemical deafferentation was induced by s.c. injections of capsaicin once daily for three consecutive days (total dose: 100 mg/kg) 2 wk before the experiment[2,5]. All capsaicin injections were performed under ether anesthesia, and the rats were pretreated with terbutaline (0.1 mg/kg, im) and aminophylline (10 mg/kg, im) to counteract the respiratory impairment associated with capsaicin injection. The effectiveness of the treatment was tested by examining the protective wiping movements of the eye.
In another experiment, wild-type mice and IP-receptor knockout mice were given HCl/ethanol po in a volume of 0.3 mL, and killed 1 hr later[17]. Then, the stomach was removed and treated with formalin, and the mucosa was examined for hemorrhagic lesions under a dissecting microscope, as described previously. In half the animals of each group, lafutidine (10 and 30 mg/kg) or capsaicin (10 mg/kg) was given po 30 min before the administration of HCl/ethanol.
Levels of PGE2 in the rat gastric mucosa and those of 6-keto PGF1α in the mouse stomach were measured 30 min after the po administration of lafutidine (10 mg) or capsaicin (10 mg/kg). In some cases, indomethacin (5 mg/kg) was given s.c. 1 h before the capsaicin. Under ether anesthesia, the stomachs were quickly removed, opened along the greater curvature, and rinsed with ice-cold saline. To separate the mucosal layer, the corpus mucosa was placed between two glass slides squeezed with a rubber band, and placed in hexane-frozen Dry Ice and acetone. These glasses were separated, and the mucosa was collected, weighed, and put in a tube containing 100% ethanol plus 0.1 mol/L indomethacin[18,19]. Then, the samples were homogenized, and centrifuged for 10 min at 12 000 g at 4 °C. The supernatant of each sample was used for the determination of PGE2 and 6-keto PGF1α levels by EIA with a PGE2- and 6-keto PGF1α-kit, respectively (Cayman Chemical Co., Ann Arbor, MI).
Human embryonic kidney 293 (HEK293) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) supplemented with 15% fetal bovine serum (FBS, JRH Biosciences, KS) and 100 IU/mL of penicillin plus 100 μg/mL of streptomycin (Gibco BRL, MD) in an atmosphere of 5% CO2 and 95% air at 37 °C. HEK293 cells were seeded on glass cover slips coated with poly-L-lysine (Sigma Chem, MO) in 35 mm dishes. The cells grown to 70%-80% confluence were transfected with 3 μg of VR1-pCR3.1-Uni plasmid using LipofectA-MINE Reagent (Gibco BRL, MD) and were cultured in DMEM for 48 hr[20]. After the medium was changed once with calcium imaging buffer (CIB) consisting of 115 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgCl2, 1.8 mmol/L CaCl2, 13.8 mmol/L D-glucose, and 20 mmol/L HEPES (adjusted to pH 7.4 with NaOH), cells were incubated with CIB containing 5 μmol/L Fura-2-AM (Dojin, Kumamoto, Japan) at room temperature for 45 min. Then, cells were washed twice with CIB. The mobilization of Ca2+ was monitored with an ARGUS-100/CA system (Hamamatsu Photonics, Hamamatsu, Japan). Dual images (340 and 380 nm excitation, 510 nm emission) were collected for 180 s. The [Ca2+]i was calculated from Fura-2 ratios by the equation described in a previous paper[20,21], and the maximal responses were determined. Capsaicin (10-8-10-6 mol/L) or lafutidine (10-4 mol/L) was added to the medium.
Drugs used were lafutidine (UCB Japan, Tokyo, Japan), capsaicin (Nakarai Tesque, Kyoto, Japan), indomethacin, capsazepine, NG-nitro-L-arginine methyl ester (L-NAME)(Sigma Chemicals, St. Louis, MO), terbutaline (BricanylR, Fujisawa, Osaka, Japan) and aminophylline (NeophyllinR, Eisai, Tokyo, Japan). Lafutidine was suspended with 0.5% carboxymethylcellulose solution (CMC: Nacalai Tesque). Capsaicin was dissolved in a Tween 80-ethanol solution (10% ethanol, 10% Tween and 80% saline, w/w: Wako, Osaka, Japan) for sc injection while it was suspended in a 0.5% CMC for topical application. Indomethacin was suspended in saline with a drop of Tween 80 (Wako, Osaka, Japan). Other agents were dissolved in saline. Each agent was prepared immediately before use and given in a volume of 0.5 mL per 100 g body weight (rat) for po or sc administration or 0.1 mL per 10 g body weight (mouse) for po administration. Control animals received saline instead of the active agent.
Data are presented as the mean ± SE for 4-6 animals per group. Statistical analyses were performed using a two-tailed Dunnett's multiple comparison test, and values of P < 0.05 were regarded as significant.
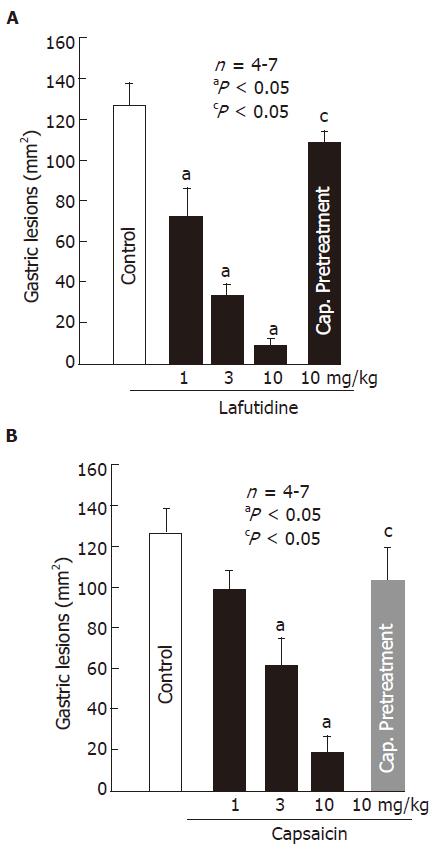
Oral administration of HCl/ethanol (60% in 150 mmol/L HCl) produced multiple lesions in the glandular mucosa, along the long axis of the rat stomach. These lesions were dose-dependently prevented by prior administration of lafutidine (1-10 mg/kg, po). A significant effect was observed even at 1 mg/kg and the inhibition was 36.8% (Figure 1A). Likewise, pretreatment of the animals with capsaicin (1-10 mg/kg, po) also provided dose-dependent protection against HCl/ethanol-induced gastric lesions and a significant effect was observed at 3 mg/kg or greater (Figure 1B). The ED50 of lafutidine and capsaicin was 1.4 mg/kg and 3.1 mg/kg, respectively. The protective effects of both lafutidine (10 mg/kg) and capsaicin (10 mg/kg) were almost totally reversed by chemical ablation of capsaicin-sensitive afferent neurons following capsaicin pretreatment (Figure 1 A and Figure 1B). Sensory deafferentation by itself did not significantly affect the development of gastric lesions in response to HCl/ethanol (data not shown).
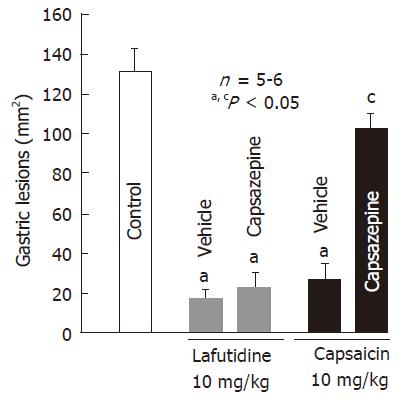
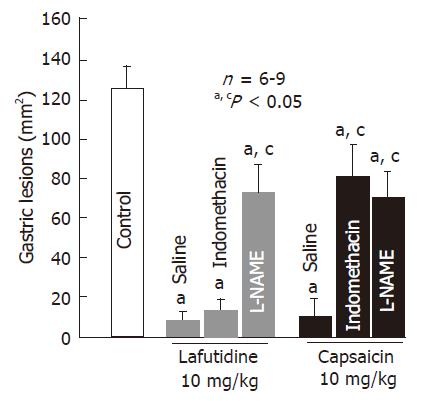
Oral administration of lafutidine or capsaicin at 10 mg/kg provided a marked protection against HCl/ethanol-induced gastric lesions in rats. The protective effect of capsaicin was significantly negated by pretreatment with capsazepine (12.5 mg/kg, po), and the degree of protection afforded by capsaicin was 19.7%, which is significantly less than that (85.4%) observed in the vehicle-treated normal rats (Figure 2). Likewise, pretreatment of the animals with indomethacin (5 mg/kg, sc) also significantly abrogated the protective action of capsaicin (Figure 3). Although capsaicin afforded significant protection against HCl/ethanol-induced gastric lesions, even in the presence of indomethacin, the degree of protection was decreased to 31.9%, which is much less than that (89.7%) observed in the saline control group. By contrast, neither capsazepine nor indomethacin significantly affected the protective action of lafutidine against HCl/ethanol-induced gastric lesions (Figure 2 and Figure 3). Lafutidine had a potent protective effect in the absence or presence of indomethacin and the degree of protection was 91.6% or 85.7%, respectively. On the other hand, the protective action of capsaicin as well as lafutidine was curtailed by prior administration of L-NAME (20 mg/kg, sc), the inhibitor of NO production. The degree of protection was 41.7% and 38.7%, respectively, and both values were significantly less than those observed in the absence of L-NAME.
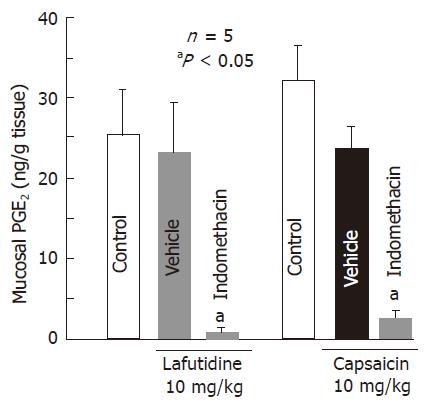
Oral administration of capsaicin (10 mg/kg) did not significantly affect the mucosal PGE2 content determined at 0.5 h after the administration (Figure 4). Likewise, the mucosal PGE2 content was not significantly altered by the po administration of lafutidine at 10 mg/kg. Prior administration of indomethacin (5 mg/kg, sc) markedly reduced the PGE2 content in the presence of capsaicin or lafutidine and the inhibition was 84.7% or 94.2%, respectively.
It was found that lafutidine, unlike capsaicin, provided gastric protection against HCl/ethanol-induced damage in rats, even in the presence of indomethacin. In addition, we previously found that endogenous PGs modulate the protective action of capsaicin through the activation of IP receptors[12]. To further investigate the involvement of IP receptors in the gastro-protection afforded by lafutidine, we examined the effect of this agent on ethanol-induced gastric lesions in both wild-type and IP receptor knockout mice, in comparison with that of capsaicin.
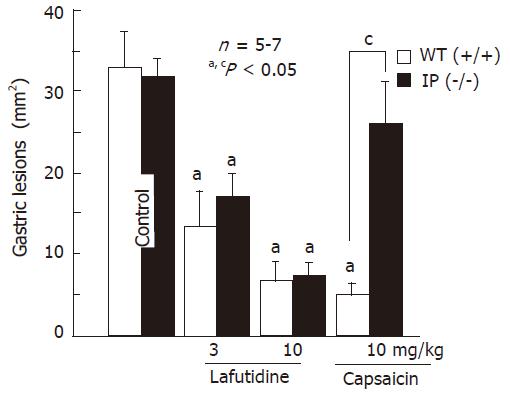
Intragastric administration of HCl/ethanol (0.3 mL) caused hemorrhagic lesions in the stomach of wild-type mice and the lesion area was 34.1 ± 4.9 mm2 (Figure 5). HCl/ethanol produced gastric lesions in IP-receptor knockout animals, similar to wild-type mice, and the severity of these lesions did not differ between two groups. Similar to the findings in rats, the severity of these lesions in wild-type mice was significantly reduced by prior administration of capsaicin (10 mg/kg, po) and the inhibition was 82.3%. Likewise, intragastric lafutidine (3 and 10 mg/kg) significantly reduced the severity of these lesions in wild-type animals in a dose-dependent manner and the degree of protection was 68.2% and 79.8%, respectively. This agent protected the stomach of IP receptor knockout mice against HCl/ethanol as effective as observed in wild-type animals. However, the capsaicin did not afford significant protection in the animals lacking IP receptors and the degree of protection was 21.4%, which is significantly less than that (82.3%) observed in wild-type mice.
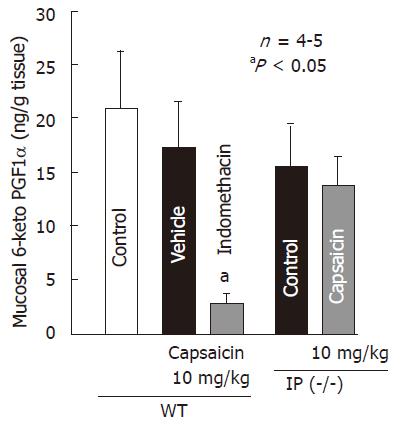
Since the protective action of capsaicin was not observed in IP-receptor knockout mice, we measured the effect of capsaicin on gastric 6-keto PGF1α production in both wild-type and IP-knockout mice. Oral administration of capsaicin (10 mg/kg) did not significantly affect 6-keto PGF1α content, same as the effect on PGE2 content in rat stomachs (Figure 6). Indomethacin (5 mg/kg, sc) markedly decreased 6-keto PGF1α content in the presence of capsaicin and the reduction was 84.1%. Similarly, capsaicin had no effect on 6-keto PGF1α production in IP-receptor knockout mice.
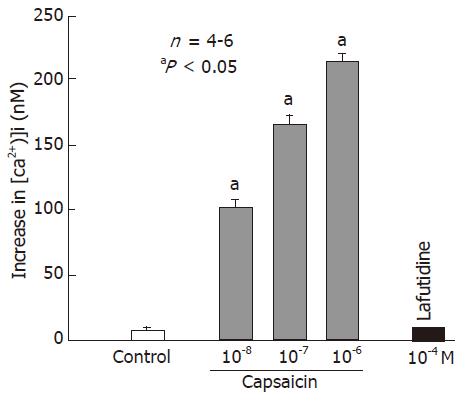
To investigate the interaction of lafutidine with VR1 receptors, we examined the effect of lafutidine on the concentration of Ca2+ in rat VR1-transfected HEK239 cells. Addition of capsaicin (10-8-10-6 mol/L) evoked a dose-dependent and significant increase in [Ca2+]i in the HEK239 cells (Figure 7). By contrast, lafutidine even at 10-4 mol/L did not effect the [Ca2+]i.
Lafutidine, a histamine H2 receptor antagonist, has been shown to afford protection in the gastrointestinal tract, partly through capsaicin-sensitive afferent neurons[16]. However, the precise mechanism of this action, including the dependence on endogenous PGs and the interaction with TRPV1, remains unknown. The present study confirmed that lafutidine protected the stomach from HCl/ethanol-induced injury through the activation of capsaicin-sensitive afferent neurons, similar to capsaicin. furthermore, we demonstrated that the action mode of these two agents differs in terms of dependency on PGs and interaction with TRPV1. It is assumed that lafutidine interacts with capsaicin-sensitive afferent neurons at yet unidentified sites other than TRPV1.
Lafutidine, an antiulcer drug developed in Japan[13], has been shown to have a potent antisecretory effect due to a blockage of histamine H2 receptors and mucosal protective action in the gastrointestinal tract including the esophagus, stomach, small intestine and large intestine[14-16,22,23]. The latter effect is independent of the antisecretory action and mediated by capsaicin-sensitive afferent neurons[14,15,22,23], because the effect was not mimicked by cimetidine, another H2-receptor antagonist, and was significantly abrogated by chemical ablation of the afferent neurons. In the present study, we also confirmed that the development of gastric lesions in response to HCl/ethanol was dose-dependently prevented by prior administration of lafutidine, and this effect was totally attenuated by sensory defunctionalization following pretreatment with a large dose of capsaicin. It is known that stimulation of capsaicin-sensitive afferent neurons releases calcitonin gene-related peptide (CGRP), and the action of CGRP is in part mediated by endogenous nitric oxide (NO)[3]. Indeed, several studies showed that the gastro-protective action of capsaicin, a selective stimulant of these afferent neurons, is attenuated by the NO synthase inhibitor L-NAME as well as the CGRP antagonist CGRP8-37[16,24]. Onodera et al[16] reported that the protective effect of lafutidine on ethanol-induced gastric lesions was also significantly mitigated by prior administration of CGRP or L-NAME, suggesting the involvement of both CGRP and NO in the protective action. We previously reported that the protective effect of lafutidine on indomethacin-induced small intestinal lesions was abrogated by pretreatment with L-NAME as well as sensory deafferentation[15,25]. The present study also showed that pretreatment of the animals with L-NAME significantly attenuated the protective action of both capsaicin and lafutidine against HCl/ethanol-induced gastric lesions.
Interestingly, the presence of endogenous PGs is required for the expression of various capsaicin-induced actions in the stomach, including the mucosal protection, the increase in mucosal blood flow, and the stimulation of bicarbonate secretion[5,26,27]. Indeed, the gastro-protective effect of capsaicin was curtailed when animals were pretreated with indomethacin to inhibit PG production[5,28]. We confirmed in this study that the protective effect of capsaicin on HCl/ethanol-induced gastric lesions was significantly mitigated in the presence of indomethacin, confirming the involvement of endogenous PGs in this action. Consistent with our previous study[12], we observed that capsaicin failed to exhibit gastro-protective action in prostacyclin IP receptor knockout mice, suggesting a modulator role for IP receptors in the facilitation by endogenous PGs of gastric protection mediated by capsaicin-sensitive afferent neurons. Recently, it has been shown that endogenous phosphatidylinositol-4, 5-bisphosphate (PtdIns(4,5)P2) inhibits TRPV1, and the repression can be alleviated by agents that activate phospholipase C (PLC)[29,30]. Bradykinin potentiates the activation of TRPV1 by capsaicin through the hydrolysis of PtdIns(4,5)P2 in a PLC-dependent manner[30]. Thus, PGs might sensitize the afferent neurons to capsaicin through IP receptors, by somehow releasing TRPV1 from PtdIns(4,5)P2-mediated inhibition. However, as shown in the present study, lafutidine exhibited a gastro-protective action against HCl/ethanol-induced damage in indomethacin-pretreated rats as well as in IP receptor knockout mice, as effective as in the respective control animals, strongly suggesting that the effect is independent of endogenous PGs. It is known that mucosal acidification increases HCO3- secretion in the stomach, and this response was attenuated by indomethacin [31]. We previously reported that lafutidine restored the response to capsaicin even in the presence of indomethacin [21]. It is thus assumed that lafutidine somehow plays a supportive role in the mechanism of action mediated by capsaicin-sensitive afferent neurons, similar to endogenous PGs.
The peptidergic afferent innervation of the stomach has been demonstrated in various species of animals including the rat [32]. Many studies including ours have shown that lafutidine affords gastric protection through capsaicin-sensitive sensory neurons, similar to capsaicin. However, it remains unknown how lafutidine interacts with these neurons. It is known that capsaicin stimulates the afferent neurons through the activation of TRPV1, resulting in the liberation of CGRP, the predominant neurotransmitter of spinal afferents in the rat stomach, and by so doing exerts a gastro-protective action [33]. This idea was supported by the present finding that the protective effect of capsaicin was totally inhibited by capsazepine, an antagonist of TRPV1. It is possible that lafutidine also interacts with TRPV1 to facilitate the activity of afferent neurons, similar to capsaicin. Nishihara et al[34], however, reported that lafutidine alone did not cause a release of CGRP from the stomach but enhanced the release induced by a submaximal dose of capsaicin. They also showed that lafutidine had no effect on the specific binding of [3H]-resiniferatoxin to TRPV1[34]. These results suggest that although lafutidine modulates the activity of capsaicin-sensitive afferent nerves, this effect is not brought about through interaction with TRPV1. We also observed that lafutidine had no effect by itself on [Ca2+]i in rat VR1-transfected HEK 239 cells, although capsaicin produced a dose-dependent increase in [Ca2+]i in these cells. Thus, it is unlikely that lafutidine modulates the activity of capsaicin-sensitive afferent neurons through interaction with TRPV1. As observed in the present study, the TRPV1 antagonist capsazepine did not significantly influence the protective effect of lafutidine, confirming that this agent does not interact with TRPV1.
As mentioned above, it was concluded that although lafutidine exhibits gastroprotective action mediated by capsaicin-sensitive neurons, similar to capsaicin, the mode of its effect differs regarding the dependency on PGs/IP receptors or TRPV1. It is assumed that lafutidine interacts with capsaicin-sensitive afferent neurons at unidentified sites other than TRPV1 and mimics the effect of endogenous PGs to modulate the activity of the afferent neurons. The exact mechanism by which lafutidine interacts with capsaicin-sensitive afferent neurons awaits further study.
We thank Professor Shu Narumiya, Kyoto University Faculty of medicine for kindly supplying IP receptor-knockout mice.
Supported in part by the Kyoto Pharmaceutical University’s “21st Century COE” program and the “Open Research” Program from the Ministry of Education, Science and Culture of Japan
S- Editor Wang J L- Editor Zhao JB E- Editor Zhang Y
| 1. | Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433-443. [PubMed] |
| 2. | Holzer P, Sametz W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology. 1986;91:975-981. [PubMed] |
| 3. | Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 333] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Takeuchi K, Niida H, Matsumoto J, Ueshima K, Okabe S. Gastric motility changes in capsaicin-induced cytoprotection in the rat stomach. Jpn J Pharmacol. 1991;55:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6360] [Cited by in RCA: 6687] [Article Influence: 238.8] [Reference Citation Analysis (0)] |
| 7. | Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159-212. [PubMed] |
| 8. | Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 874] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 9. | Ohishi S, Ueno A, Matsumoto H, Murata T, Ushikubi F, Narumiya S. Evidence for involvement of prostaglandin I2 as a major nociceptive mediator in acetic acid-induced writhing reaction: a study using IP-receptor disrupted mice. Adv Exp Med Biol. 1999;469:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ueno A, Naraba H, Ikeda Y, Ushikubi F, Murata T, Narumiya S, Oh-ishi S. Intrinsic prostacyclin contributes to exudation induced by bradykinin or carrageenin: a study on the paw edema induced in IP-receptor-deficient mice. Life Sci. 2000;66:PL155-PL160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Boku K, Ohno T, Saeki T, Hayashi H, Hayashi I, Katori M, Murata T, Narumiya S, Saigenji K, Majima M. Adaptive cytoprotection mediated by prostaglandin I(2) is attributable to sensitization of CRGP-containing sensory nerves. Gastroenterology. 2001;120:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Takeuchi K, Kato S, Takeeda M, Ogawa Y, Nakashima M, Matsumoto M. Facilitation by endogenous prostaglandins of capsaicin-induced gastric protection in rodents through EP2 and IP receptors. J Pharmacol Exp Ther. 2003;304:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Shibata M, Yamaura T, Inaba N, Onodera S, Chida Y, Ohnishi H. Gastric antisecretory effect of FRG-8813, a new histamine H2 receptor antagonist, in rats and dogs. Eur J Pharmacol. 1993;235:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Onodera S, Shibata M, Tanaka M, Inaba N, Yamaura T, Ohnishi H. Gastroprotective activity of FRG-8813, a novel histamine H2-receptor antagonist, in rats. Jpn J Pharmacol. 1995;68:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kato S, Tanaka A, Kunikata T, Umeda M, Takeuchi K. Protective effect of lafutidine against indomethacin-induced intestinal ulceration in rats: relation to capsaicin-sensitive sensory neurons. Digestion. 2000;61:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Onodera S, Nishida K, Takeuchi K. Unique profile of lafutidine, a novel histamine H2-receptor antagonist: mucosal protection throughout gastrointestinal tract mediated by capsaicin-sensitive afferent neurons. Curr Pharm Design on line. 2004;1:133-144. |
| 17. | Araki H, Ukawa H, Sugawa Y, Yagi K, Suzuki K, Takeuchi K. The roles of prostaglandin E receptor subtypes in the cytoprotective action of prostaglandin E2 in rat stomach. Aliment Pharmacol Ther. 2000;14 Suppl 1:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 638] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Takeuchi K, Araki H, Umeda M, Komoike Y, Suzuki K. Adaptive gastric cytoprotection is mediated by prostaglandin EP1 receptors: a study using rats and knockout mice. J Pharmacol Exp Ther. 2001;297:1160-1165. [PubMed] |
| 20. | Tsutsumi S, Tomioka A, Sudo M, Nakamura A, Shirakura K, Takagishi K, Kohama K. Propofol activates vanilloid receptor channels expressed in human embryonic kidney 293 cells. Neurosci Lett. 2001;312:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Mimaki H, Kagawa S, Aoi M, Kato S, Satoshi T, Kohama K, Takeuchi K. Effect of lafutidine, a histamine H2-receptor antagonist, on gastric mucosal blood flow and duodenal HCO3- secretion in rats: relation to capsaicin-sensitive afferent neurons. Dig Dis Sci. 2002;47:2696-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Nagahama K, Yamato M, Kato S, Takeuchi K. Protective effect of lafutidine, a novel H2-receptor antagonist, on reflux esophagitis in rats through capsaicin-sensitive afferent neurons. J Pharmacol Sci. 2003;93:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Okayama M, Tsubouchi R, Kato S, Takeuchi K. Protective effect of lafutidine, a novel histamine H2-receptor antagonist, on dextran sulfate sodium-induced colonic inflammation through capsaicin-sensitive afferent neurons in rats. Dig Dis Sci. 2004;49:1696-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Peskar BM. Neural aspects of prostaglandin involvement in gastric mucosal defense. J Physiol Pharmacol. 2001;52:555-568. [PubMed] |
| 25. | Tanaka A, Mizoguchi H, Hase S, Miyazawa T, Takeuchi K. Intestinal protection by lafutidine, a histamine H(2)-receptor antagonist, against indomethacin-induced damage in rats--role of endogenous nitric oxide. Med Sci Monit. 2001;7:869-877. [PubMed] |
| 26. | Matsumoto J, Takeuchi K, Ueshima K, Okabe S. Role of capsaicin-sensitive afferent neurons in mucosal blood flow response of rat stomach induced by mild irritants. Dig Dis Sci. 1992;37:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Aihara E, Hayashi M, Sasaki Y, Kobata A, Takeuchi K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: comparison with mucosal acidification. J Pharmacol Exp Ther. 2005;315:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Uchida M, Yano S, Watanabe K. The role of capsaicin-sensitive afferent nerves in protective effect of capsaicin against absolute ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1991;55:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 638] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 30. | Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 969] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 31. | Takeuchi K, Ueshima K, Matsumoto J, Okabe S. Role of capsaicin-sensitive sensory nerves in acid-induced bicarbonate secretion in rat stomach. Dig Dis Sci. 1992;37:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Sternini C, Reeve JR, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987;93:852-862. [PubMed] |
| 33. | Merchant NB, Dempsey DT, Grabowski MW, Rizzo M, Ritchie WP Jr. Capsaicin-induced gastric mucosal hyperemia and protection: the role of calcitonin gene-related peptide. Surgery. 1994;116:419-425. [PubMed] |
| 34. | Nishihara K, Nozawa Y, Nakano M, Ajioka H, Matsuura N. Sensitizing effects of lafutidine on CGRP-containing afferent nerves in the rat stomach. Br J Pharmacol. 2002;135:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |