Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.3015
Revised: November 2, 2005
Accepted: November 10, 2005
Published online: May 21, 2006
AIM: To explore the relationship among interferon-γ (IFN-γ) activity, fibrogenesis, T cell immune responses and hepatic inflammatory activity.
METHODS: Peripheral blood samples from a total of 43 hepatitis B cirrhotic patients (LC) and 19 healthy controls (NC) were collected to measure their serum levels of IFN-γ, interleukin-2 (IL-2), soluble interleukin-2 receptor (sIL-2R), interleukin-10 (IL-10) and three serological markers of fibrosis including hyaluronic acid (HA), procollagen type III peptide (PIIIP), and type IV collagen were measured using a double antibody sandwich ELISA. Also, serum total bilirubin (TB) and alanine aminotransferase (ALT) were measured by routine measures.
RESULTS: The concentrations of serological markers of fibrosis in patients with active cirrhosis (ALC) were significantly higher than those in stationary liver cirrhosis (SLC) or NC groups. The levels of serological markers in HBeAg-positive patients were significantly higher than those in HBeAg-negative patients. In SLC and ALC patients, a negative linear correlation was found between IFN-γ levels and the serological markers of fibrosis. IFN-γ and IL-2 levels in the ALC group were significantly higher than those in the SLC and NC groups, but the statistical difference was not significant between the latter two. In contrast, IL-10 levels in the SLC group were significantly higher than that in the NC group, but no significant difference was found between SLC and ALC groups. The sIL-2R level was elevated gradually in all these groups, and the differences were significant. Positive linear correlations were seen between IFN-γ activity and ALT levels (r = 0.339, P < 0.05), and IL-2 activity and TB levels (r = 0.517, P < 0.05). sIL-2R expression was positively correlated with both ALT and TB levels (r = 0.324, 0.455, P < 0.05), whereas there was no statistically significant correlation between IL-10 expression and serum ALT and TB levels (r = -0.102, -0.093, P > 0.05). Finally, there was a positive correlation between IFN-γ and IL-2 levels.
CONCLUSION: T cell immune responses are correlated with fibrosis and hepatic inflammatory activity and may play an important role in liver cirrhosis.
- Citation: Tang JT, Fang JY, Gu WQ, Li EL. T cell immune response is correlated with fibrosis and inflammatory activity in hepatitis B cirrhotics. World J Gastroenterol 2006; 12(19): 3015-3019
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/3015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.3015
In Chinese population, infection with the hepatitis B virus is the major cause of liver injury. Inflammation stimulates hepatic stellate cells, which have been shown to be involved in fibrogenesis. Liver fibrosis is primarily due to an imbalance of proliferation and degradation of the extracellular matrix (ECM) triggered by hepatocytic necrosis and inflammation. Liver fibrosis is characterized by a frequent release of cytokines, such as transforming growth factor β1 (TGF-β1), platelets-derived growth factor (PDGF), tumor necrosis factor α (TNF-α), IL-6, interferon-γ (IFN-γ), hepatocyte growth factor (HGF), insulin-like growth factor (IGF) and IL-10[1]. Currently, the serum markers of fibrosis are hyaluronic acid (HA), procollagen type III peptide (PIIIP), and type IV collagen. These markers are used to reflect the activity of hepatic fibrotic process[2].
The relationship among immune response, viral clearance, inflammatory process, and liver fibrosis is very complicated. IFN-γ is not only an anti-fibrotic agent, but also a Th1-type cytokine, which participates in the immune response involved in fibrogenesis, as well as the inflammatory process. Thus, the role of T cell immune response in hepatic fibrosis is not clear.
The goal of this study was to characterize the relationship among T cell immune responses, inflammatory activity and liver fibrosis. Thus, we assayed serum levels of IFN-γ and other T cell-type cytokines and serological markers of fibrosis in patients with varying degrees of liver inflammation stemming from hepatitis B cirrhotics.
Forty-three patients with LC (18 females and 25 males with a mean age of 58.4 years) and 19 normal healthy adults (NC) (9 females and 10 males with a mean age of 51.4 years) were enrolled in this study. The patients with LC were all diagnosed by the same pathologist and classified according to WHO’s histological classification of LC. Among them, six patients were HBeAg-positive. The patients were confirmed to have moderate hepatic fibrosis pathology, according to the Scheuer and Chevallier scoring system[3,4], stemming from chronic hepatitis B for 6 months to 30 years and none had received any form of antiviral therapy. Twenty-three of the cases were placed in the stationary LC (SLC) group and twenty cases in the active LC (ALC) group. Group designations were made according to the “2000 National Prevention and Treatment Plan of Viral Hepatitis”, in which inflammation grades (G) were classified as G0, G1-2, G3 and G4 for no inflammation, mild, moderate and severe inflammation, respectively. SLC was defined as LC patients with normal level of aminotransferase, no obvious jaundice and no or mild inflammation according to pathologic inflammation grade. ALC was defined as LC patients with elevated aminotransferase and bilirubin, and moderate or severe inflammation according to pathologic inflammation grade. The clinical pathological features of each patient were reviewed and are shown in Table 1. Written informed consent was obtained from all subjects before study entry.
| According to state of illness | According to virus replication | ||
| Group | n | Group | n |
| SLC | 23 | HBeAg (+) | 6 |
| ALC | 20 | HBeAg (-) | 37 |
Peripheral whole blood samples were collected from patients and normal controls and serum was frozen at -70 °C until used.
We measured the serum levels of IFN-γ, IL-2, sIL-2R and IL-10 as well as HA, PIIIP, IV-C by the double antibody sandwich ELISA. The IFN-γ assay kit was provided by the American R&D Corporation, IL-2 detecting reagents were purchased from the French Diaclone Corporation, and the kits for sIL-2R and IL-10 were obtained from Beijing Jinmei Biotech, Beijing. HA, PIIIP and collagen IV were determined using kits from Shanghai Maoyuan Biotech, Shanghai. Assays were performed following the manufacturer’s instructions. Optical density of each well was determined within 30 min, using a microplate reader set at 450 nm.
Serum total bilirubin (TB) and alanine aminotransferase (ALT) were measured by routine methods.
All biochemical data were expressed as mean ± SD. SPSS PC+ software package was used to analyzed differences between groups for statistical significance using t tests. Differences were considered significant when P values were < 0.05. Additionally, Pearson’s correlation coefficients were calculated.
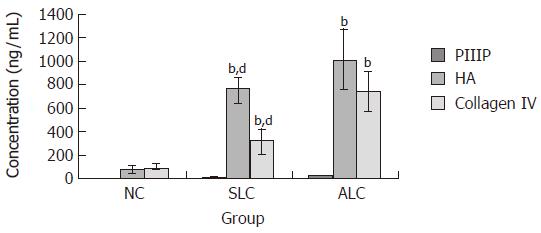
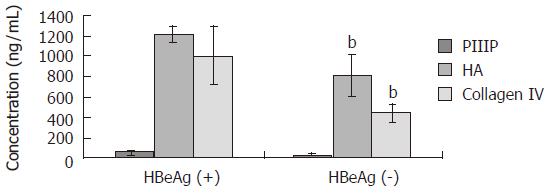
Figure 1 shows the concentrations of serological markers of fibrosis in NC, SLC and ALC patients [PIIIP: 2.322 ± 2.209, 16.730 ± 11.142 and 41.315 ± 20.635 ng/mL; HA: 79.247 ± 28.531, 752.870 ± 114.838 and 1 013.300 ± 252.988 ng/mL; type IV collagen: 101.974 ± 27.855, 323.661 ± 104.021 and 747.950 ± 160.929 ng/mL (bP < 0.01 for all)]. The concentrations of serological markers of fibrosis in the ALC group patients were significantly higher than those in the SLC or NC groups. In addition, levels of serologic markers of fibrosis were significantly higher in HbeAg-positive patients as compared with the HBeAg-negative patients [PIIIP: 49.774 ± 21.831 vs. 24.665 ± 17.996 ng/mL; HA: 1 207.650 ± 79.832 vs. 819.866 ± 203.495 ng/mL; type IV collagen: 1 007.614 ± 286.012 vs. 442.105 ± 84.827 ng/mL (bP < 0.01 for all, Figure 2)].
A negative linear correlation was observed between serum IFN-γ levels and serological markers of fibrosis HA, PIIIP and type IV collagen in patients with LC. The correlation coefficients (r) of HA, PIIIP and type IV collagen with IFN-γ are listed in Table 2.
| SLC | ALC | |||||
| PIIIP | HA | Collagen IV | PIIIP | HA | Collagen IV | |
| Correlation coefficient (r) with IFN- γγ | -0.531b | -0.523a | -0.599b | -0.482a | -0.499a | -0.578b |
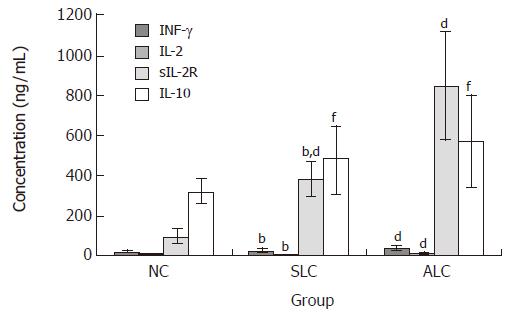
Figure 3 shows IFN-γ,IL-2, sIL-2R and IL-10 levels in NC, SLC and ALC groups (IFN-γ: 21.213 ± 5.876, 23.619 ± 8.183 and 35.881 ± 9.032 pg/mL; IL-2: 5.404 ± 2.161, 6.003 ± 2.174 and 11.989 ± 7.232 pg/mL; IL-10: 320.684 ± 59.864, 480.455 ± 169.554 and 570.500 ± 230.451 pg/mL; sIL-2R: 94.917 ± 33.672, 380.571 ± 92 and 848.656 ± 268.105 pg/mL). IFN-γ and IL-2 levels in the ALC group were significantly higher than those in the SLC and NC groups (P < 0.01), but no significant difference between the SLC and NC groups was observed. IL-10 levels in the SLC group were significantly higher than that in the NC group (P < 0.01), likewise, there was no significant difference between the SLC and ALC groups. sIL-2R levels elevated gradually in all three groups, and the differences were significant (P < 0.01).
Positive linear correlations were found between IFN-γ activity and ALT levels (r = 0.339, P < 0.05) and IL-2 activity and TB levels (r = 0.517, P < 0.05; Table 3). sIL-2R expression was significantly positively correlated with both ALT and TB levels (r = 0.324, 0.455, P < 0.05). But the correlation between IL-10 expression and ALT and TB levels was not significant (r = -0.102, -0.093, P > 0.05). Furthermore, a positive correlation between IFN-γ and IL-2 levels (r = 0.324, P < 0.05) was observed.
| IFN-γ | IL-2 | sIL-2R | IL-10 | |
| ALT | r = 0.339a | r = 0.119 | r = 0.324a | r = -0.102 |
| TB | r = 0.287 | r = 0.517a | r = 0.455a | r = -0.093 |
| IFN-γ | r = 0.324a | r = 0.110 | r = 0.183 | |
| IL-2 | r = 0.220 | r = 0.018 | ||
| sIL-2R | r = 0.086 |
Liver fibrosis is a common feature in chronic liver disease, which is relevant to inflammation, viral replication and T cell immune responses. T helper (Th) cells are important in T cell immune responses and are divided into Th1, Th2 and Th0 cells characterized by different cytokines they secrete. Th1 cells are characterized by IFN-γ, IL-2 and TNF-β production, whereas Th2 cells are characterized by IL-10, IL-4, IL-5 and IL-6 production. The cytokines produced by Th1 and Th2 cells can influence and restrain each other.
The present study showed that IFN-γ levels were remarkably high in the ALC group and positively correlated with ALT levels. In fact, several studies have shown that IFN-γ is associated with elevation of ALT levels in chronic hepatitis B patients[5,6]. This might be due to different immune responses in chronic versus acute infections.
Using IFN-γ and IL-2 levels to report the functional state of Th1 cells and IL-10 levels to report the functional state of Th2 cells in patients with LC, we found that Th1 cells predominated in the ALC group, while Th2 cells predominated in the SLC group. Recently, an imbalance of Th1/Th2 cells was found in HBV-infected chronic patients, and T cells were activated in the patients with chronic hepatitis B[7]. The majority of peripheral blood mononuclear cells shown in hepatitis B patients were Th0 cells, but the proportion of Th1 cells increased remarkably along with the hepatic inflammatory activity[8]. Th1-type cytokines levels were higher in patients with serious inflammation than those with milder inflammation. Our results also showed that there was no significant difference in IFN-γ or IL-2 levels between SLC and NC groups, thereby suggesting that Th1-type cytokines decrease with alleviation of inflammation.
An imbalance of Th1 and Th2 cells exists in patients with LC. Our results indicated a predominance of IFN-γ, IL-2 and sIL-2R in LC patients with active inflammation and a predominance of Th2-type cytokine IL-10 in ALC patients. Detection of serum levels of these cytokines might provide insight into the occurrence of liver inflammation, immune response and outcome of the patients.
IFN-γ plays a crucial role in modulating immune responses for HBV-induced cirrhosis[9-12]. Our study showed that serum IFN-γ levels were negatively correlated with serologic markers of fibrosis in ALC and SLC patients, suggesting that IFN-γ may play a role in the fibrogenesis process in patients with LC. Many scholars hypothesize that there are two mechanisms involved in the anti-fibrotic role of IFN-γ. One role is to lower the mRNA expression level of precollagen I, III in fibroblasts. Sakaida et al[10] found a decrease in precollagen I, III levels and myofibroblasts in a rat liver fibrosis model, after giving the rats IFN-γ (50 000 U) daily. A second role is to inhibit activation and proliferation of hepatic stellate cells. In IFN-γ-treated animals, activation of hepatic stellate cells induced by liver injury was reduced, as shown by the decrease of proliferating hepatic stellate cells and the reduction of parenchymal area occupied by smooth muscle-specific alpha-actin (α-SMA)-positive cells. In addition, there may be some other explanations. For example, IFN-γ can cause changes in the expression of Bcl-2 and Bax genes in liver fibrosis in rats. Additionally, IFN-γ may promote myofibroblasts apoptosis and inhibit ECM synthesis[13]. Results from this study showed that levels of serologic markers of fibrosis were much higher in ALC than in SLC patients. Additionally, our results showed significantly higher levels of serologic markers of fibrosis in HBeAg-positive as compared with HBeAg-negative patients. Taken together, the data suggest that inflammation and viral replication promote the process of fibrosis.
HBV activates T cells, B cells and macrophages to express IL-2R with high competency. It has been proposed that sIL-2R as well as IL-2 might cause hepatic injury in chronic active hepatitis C[14]. sIL-2R levels decreased substantially after IFN treatment in patients with chronic hepatitis C. Consistent with our results, Sawayama et al[15] reported an increase of sIL-2R levels in LC patients and a positive correlation between IL-2 and ALT levels. The reason for the sIL-2R increase is still unknown, but it may be due to changes in cytokine clearance in the liver. On the other hand, activated T cells can promote the production of sIL-2R and may therefore augment serum sIL-2R levels.
A low IL-10 level could lead to an increase in pro-inflammatory cytokines and a worse prognosis. But in this study, we found non- significant correlation between IL-10 expression and ALT and TB levels (r = -0.102, -0.093, P > 0.05). In fact, an imbalance of IL-10 and IFN-γ may play an important role in promoting inflammatory reactions leading to massive liver damage[16,17], since excessive IL-10 secretion would restrain the immune response against the virus. This imbalance may thus lead to a sustained hepatitis B viral infection[5,18-20].
Because of the existence of interactions between IFN-γ and IL-2 in the immune response, we examined IL-2 levels in the LC patients. Our results showed that IL-2 levels in the ALC group were significantly higher than those in the SLC or NC groups, but IL-2 levels in the SLC and NC groups did not differ significantly. A positive correlation was found between IFN-γ and IL-2 levels as well as between IL-2 and TB levels.
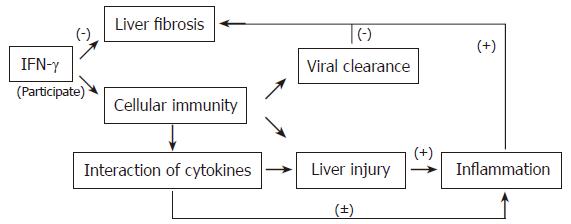
In conclusion, IFN-γ ameliorates liver fibrogenesis and also participates in the cellular immune response as an important Th1-type cell, which enhances the action of cytolytic T cell (CTL) to clear HBV and slows the progression of fibrosis (Figure 4). However, Th1-type cellular immunoresponses can cause liver injury during viral clearance. Inflammatory cells are recruited to the sites where hepatocytic necrosis has occurred and initiates and augments liver fibrosis. Thus, when IFN-γ is given as a treatment for LC patients during an active stage, the pro-inflammatory role of IFN-γ must be considered. At the Annual meeting of the Americans for the Study of Liver Disease (AASLD), IFN-γ has been formally recommended to be the preferred anti-fibrotic drug. Our results indicate that there still remain many problems to be solved in the course of treatment, such as IFN-γ dosage, and how to assess the efficacy of the treatment, as linked to the stage of fibrosis, inflammatory processes and serum concentration levels of IFN-γ in those patients. However, our results are important for evaluation and discussion of the feasibility of IFN-γ use in treating patients with liver cirrhosis.
S- Editor Wang J L- Editor Kumar M E- Editor Ma WH
| 1. | Girón-González JA, Martínez-Sierra C, Rodriguez-Ramos C, Macías MA, Rendón P, Díaz F, Fernández-Gutiérrez C, Martín-Herrera L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004;24:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Tsukamoto T, Yamamoto T, Ikebe T, Takemura S, Shuto T, Kubo S, Hirohashi K, Kinoshita H. Serum markers of liver fibrosis and histologic severity of fibrosis in resected liver. Hepatogastroenterology. 2004;51:777-780. [PubMed] |
| 3. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1506] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 4. | Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Hyodo N, Tajimi M, Ugajin T, Nakamura I, Imawari M. Frequencies of interferon-gamma and interleukin-10 secreting cells in peripheral blood mononuclear cells and liver infiltrating lymphocytes in chronic hepatitis B virus infection. Hepatol Res. 2003;27:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Lee M, Lee M, Lee SK, Son M, Cho SW, Park S, Kim HI. Expression of Th1 and Th2 type cytokines responding to HBsAg and HBxAg in chronic hepatitis B patients. J Korean Med Sci. 1999;14:175-181. [PubMed] |
| 7. | Anastassakos C, Alexander GJ, Wolstencroft RA, Avery JA, Portmann BC, Panayi GS, Dumonde DC, Eddleston AL, Williams R. Interleukin-1 and interleukin-2 activity in chronic hepatitis B virus infection. Gastroenterology. 1988;94:999-1005. [PubMed] |
| 8. | Priĭmiagi LS, Tefanova VT, Tallo TG, Shmidt EV, Solomonova OV, Tuĭsk TP, Kikosh GV, Krupskaia LM, Lisitsyna SA. [Th1-cytokines in chronic hepatitis B and C]. Vopr Virusol. 2002;47:23-27. [PubMed] |
| 9. | Xie Y, Zhao H, Dai WS, Xu DZ. HBV DNA level and antigen concentration in evaluating liver damage of patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2003;2:418-422. [PubMed] |
| 10. | Sakaida I, Uchida K, Matsumura Y, Okita K. Interferon gamma treatment prevents procollagen gene expression without affecting transforming growth factor-beta1 expression in pig serum-induced rat liver fibrosis in vivo. J Hepatol. 1998;28:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Baroni GS, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 204] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Tao J, Cai WM, Lu LW, Chen QW, Weng HL, Zhang YS. [Expression of Bcl-2 and Bax proteins in rat liver fibrosis model and the role of IFN-gamma]. Zhonghua Gan Zang Bing Za Zhi. 2003;11:669-672. [PubMed] |
| 14. | Simsek H, Kadayifci A. Serum interleukin 2 and soluble interleukin 2 receptor in chronic active hepatitis C: effect of interferon therapy. J Int Med Res. 1996;24:239-245. [PubMed] |
| 15. | Sawayama Y, Hayashi J, Kawakami Y, Furusyo N, Ariyama I, Kishihara Y, Ueno K, Kashiwagi S. Serum soluble interleukin-2 receptor levels before and during interferon treatment in patients with chronic hepatitis B virus infection. Dig Dis Sci. 1999;44:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology. 2002;36:1001-1008. [PubMed] |
| 17. | Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin Exp Immunol. 2004;135:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kato M, Ikeda N, Matsushita E, Kaneko S, Kobayashi K. Involvement of IL-10, an anti-inflammatory cytokine in murine liver injury induced by Concanavalin A. Hepatol Res. 2001;20:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Louis H, Le Moine O, Goldman M, Devière J. Modulation of liver injury by interleukin-10. Acta Gastroenterol Belg. 2003;66:7-14. [PubMed] |
| 20. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |