INTRODUCTION
Hepatitis B virus (HBV) infection is widespread in humans, with an estimated 400 million persons infected globally and an annual death rate of 1.2 million[1]. HBV is a small DNA virus with a 3.2 kb genome and belongs to the family Hepadnaviridae which also includes related hepatotrophic viruses from other animal species[2]. This partially double stranded genome contains four overlapping open reading frames (ORFs) that encode the surface (S, including the pre-S regions), core (C, including the pre-core region), polymerase (P), and X proteins. The HBV DNA genome replicates by a unique mechanism that involves an RNA intermediate, called the pregenomic RNA (pgRNA). The viral relaxed circular (rc) DNA genome is released in the host cytosol, migrates to the nucleus, and is converted into a covalently closed circular DNA (cccDNA) form that serves as the template for all viral transcripts. One of the major transcripts, the pgRNA is reverse transcribed into a single-stranded DNA (ssDNA) form that is the hallmark of viral replication. This first (+) strand serves as template for the viral polymerase to further synthesize the second, comparatively short (-) strand to become a partial double stranded circle (rcDNA), a minor population of which remains as a double stranded linear DNA (dslDNA) in special cases[3].
One major hurdle in the study of HBV molecular biology, its pathogenesis and the development of new antiviral agents, is the lack of suitable in vitro or small animal models of chronic HBV (CHB) infection in humans. Although the use of nucleos(t)ide analogs could potentially change the course of HBV infection due to the emergence of drug-resistant mutants, novel nucleos(t)ide analog-based drugs have opened up new frontiers and challenges in the treatment of CHB. Currently, combination chemotherapy with more than one nucleo(t)side analogs are being evaluated as a new approach towards management of CHB[4]. For many years, recombinant interferon-alpha (IFN-α) has been the only effective therapeutic agent able to provide long-term disease remission for some CHB patients. However, IFN-α treatment produces significant side effects such as fatigue, fever, muscular aches, bone marrow suppression, psychosis and autoimmune conditions[5]. Although many studies have examined a combination of IFN-α and lamivudine for HBeAg-positive CHB, published trials of combination therapy provide little support for IFN-α/lamivudine combination therapy over monotherapy[6-8].
The class II interferon, IFN-γ has been used to treat hepadnaviral infections including HBV, either alone or in combination with other IFNs[9-12]. In vitro studies using Hep-HB107 cell lines, have shown IFN-γ to inhibit HBV replication[13]. In pilot studies, IFN-γ was shown to be effective in suppressing HBV replication in a proportion of Caucasian patients with chronic HBV infection[14-16]. Very recently, IFN-γ associated antiviral effects have been shown in woodchuck HBV (WHBV) infected woodchuck primary hepatocytes, in vitro as well as in vivo in neonates[17]. Studies have shown that analogous to other interferons, IFN-γ alone has minimal inhibitory effects on serum HBV levels[12,15]. The emergence of HBV mutants resistant to lamivudine[18] and other novel nucleoside analogs such as adefovir dilivoxil[19], famcyclovir[20] and entecavir[21] , as well as reports of non-response to IFN-α therapy[22] further limits the use of these agents.
Therefore, in this study we evaluated the antiviral potential of IFN-γ on the kinetics of HBV genome replication, as well as its efficacy in combination with lamivudine. For this, we used a recombinant baculovirus-HBV (Bac-HBV)/HepG2 culture system, similar to that developed earlier by Delaney et al[23]. Our results showed that sequential treatment with lamivudine and IFN-γ leads to effective suppression of HBV gene expression and genome replication.
MATERIALS AND METHODS
Cell lines and antiviral agents
HepG2 cells were obtained from ATCC and were maintained in complete DMEM supplemented with 100 mL/L FCS, 10g/L DMSO and 5 mg/L insulin (Sigma, St. Louis, MO, USA) at 37 °C in a humidified incubator supplied with 50 mL/L CO2. Infected cells were maintained in DMEM supplemented with 100 mL/L FCS, 10g/L DMSO and 50 μmol/L hydroxycortisone hemisuccinate (Sigma, St. Louis, MO, USA). Recombinant human IFN-γ expressed in E. coli, purified and optimized for bioactivity was kindly provided by Dr. Navin Khanna (ICGEB, New Delhi). The specific activity was 3 × 1010 U/g protein and the preparation was kept in 10 mmol/L PBS, 50 mmol/L NaCl, pH 6.0 at -20 °C. Lamivudine (3TC) was provided by Dabur Research Laboratories, Sahibabad (U.P.), India, and was kept as a 1 mmol/L stock in DMSO at -20 °C.
Construction of recombinant HBV-baculoviruses
A 1.6-times unit-length HBV genome (5.0 kb), constructed from the parent HBV subtype-ayw infectious clone pTKHH2 (a kind gift from Dr. Angeline Barthelomeuz, Victorian Infectious Diseases Reference Laboratory, Melbourne, Australia) was cloned into the baculovirus transfer vector, pBacPAK9 (Clontech, CA, USA) between XhoI and BglII sites, opposite to the polyhedrin promoter (pPol) (Figure 1). The resulting pBacHep1.6 plasmid was cotransfected with Bsu36I-digested Autographa californica major nuclear polyhedrosis virus (AcMNPV) DNA into the cultured Sf21 insect cell line. The transfection and recombinant baculovirus selection procedures were followed as per the manufacturer’s instruction. Viral plaques in the primary screen were selected by slot blot DNA hybridization with a 32P-labeled HBV DNA probe. In the secondary screening, baculoviral DNA was subjected to PCR with HBV S-ORF primers. Finally, the selected recombinant Bac-HBV plaques were screened by PCR for all the HBV open reading frames. The HBV ORF-specific PCR primer sets used are as follows: C-ORF: sense, GCGGGATCCACTGTTCAAGCCTCCAAGCT; antisense, CGGAG GCGAGGGAGTTCTTCTTCT; S-ORF: sense, GCGGGATCCGGACTGGGGACC ATGTGACGAAC; antisense, GCGAAGCTTGTTAGGGTTTAAATGTATACCC; P-ORF: sense, GAAGATCTCAATCTCGGGAATCTC; antisense, GTTCGTCACAGGGTCCCCCAGTCC; X-ORF: sense, TGCCAACTGGATCCTTCGCGGGACGTCCT; antisense, GCGAAGCTTAAGGAAAGAAGTCAGAAGG.
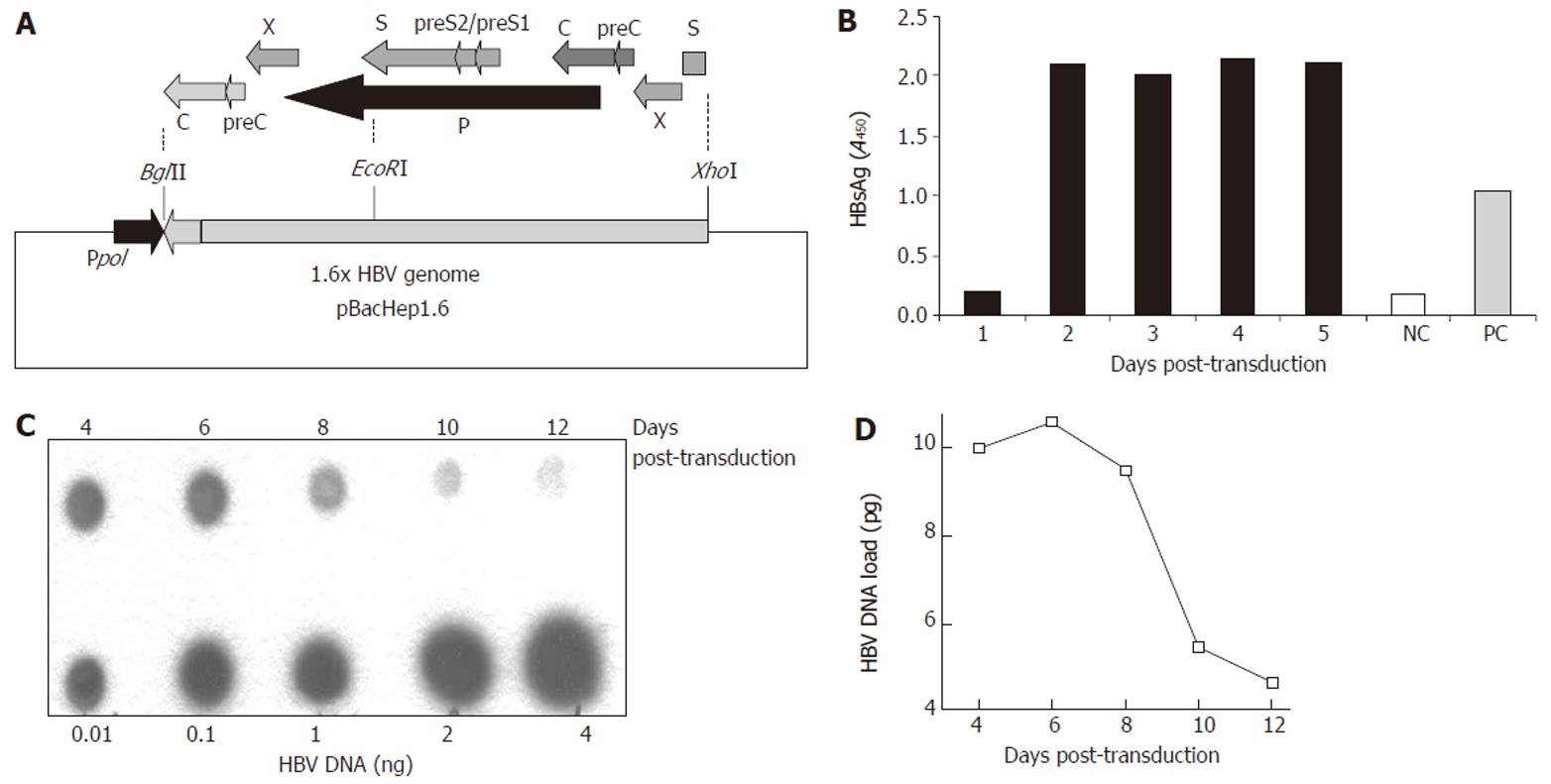
Figure 1 Construction of the recombinant Baculo-HBV virus (Bac-HBV).
A: The recombinant transfer vector, pBacHep1.6 (10.5 kb) together with the 1.6 × genome length HBV cloned in an orientation opposite to the polyhedrin promotor (pPol) in vector pBacPAK9. The construct contains HBV-ORFs: PreS/S (1x), X (2x), P (1x), PreC/C (2x) and Enh I (2x), Enh II (2x) elements; B: HBsAg expression in culture medium upon transduction with 100 moi of Bac-HBV; C: Dot blot analysis of total HBV DNA in HepG2 cells transduced with 100 moi of Bac-HBV. Different amounts of HBV DNA were used as quantitation standards, as indicated; D: Densitometric analysis and quantitative representation of the results shown in C.
A high-titre stock of selected recombinant HBV-baculoviruses was prepared from 0.5 to 1.0 litre of infected Sf21 culture supernatant. The culture supernatant was cleared at 6000 r/min for 10 min and further centrifuged in a Ti45 rotor (Beckman) at 25 000 r/min for 45 min at room temperature. A bluish-white pellet seen at the bottom of the tubes was resuspended in 3 mL PBS. This was then gently overlaid on top of a 5% (3 mL) and 40% (30 mL) sucrose cushion prepared in PBS, followed by centrifugation in an SW28 rotor (Beckman) at 18000 r/min for 30 min. A band of virus particles from the 5%-40% sucrose interface was harvested and washed with 30 mL of sterile PBS by ultracentrifugation. Finally, the virus pellet was resuspended in 1 ml of sterile PBS and stored as 200 μL aliquots at -20 °C. A working aliquot of the virus was kept at 4 °C. The titer of the virus so prepared was determined by plaque assay on Sf21 cells to be in the range of 1010 pfu/mL.
Viral transduction and drug treatment of cultured cells
HepG2 cells were seeded in 12-well plates (0.5 × 106 cells/well) or 60 mm dishes (1.5 × 106 cells/dish) one day before transduction. On the day of transduction, the medium was discarded and the cells were washed twice with PBS and once with serum-free medium. Stock recombinant Bac-HBV was diluted in serum-free medium to a final multiplicity of infection (m.o.i.) of 100 in either 150 μL (for each well of 12-well plate) or in 300 μL (for each 60 mm dish), overlaid on the cells and incubated at 37 °C in a CO2 incubator for 1-1.5 h with gentle rocking every 15 min. The infectious medium was then replaced with fresh medium and the cells incubated for the desired period with media changes every alternate day. Antiviral drugs, either alone or in combination were added on d 2 post-transduction, or as indicated for different experiments, after analysis of the baseline HBsAg expression in the culture supernatants for productive HBV infection. The drug-containing medium was replaced every alternate day throughout the study period. The doses of antiviral agents were first tested in the HepG2.2.15 cell line, prior to their use in Bac-HBV transduced HepG2 cells. Every condition was set up in at least duplicate (sometimes triplicate) wells or plates.
Analysis of HBsAg and HBeAg expressions
The secreted HBsAg and HBeAg in the culture supernatants were analyzed by the use of commercial enzyme immunoassays: HBsAg microelisa (Organon Teknika, The Netherlands) and HBeAg EIA (DiSiron SPA, Italy).
Isolation of HBV DNA from infected cells
Total and cccDNA forms of HBV DNA were isolated essentially as described elsewhere[24]. For total DNA isolation, cells were washed twice with cold PBS and lysed in 1 ml of cold lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L EDTA, 150 mmol/L NaCl, 10 g/L SDS) on ice for 10 min. The lysate was collected in an eppendorf tube, and 100 μL of proteinase K (10 g/L) was added, mixed well and incubated at 37 °C for 4 h. Following phenol-chloroform-isoamyl alcohol extraction, the DNA was precipitated with chilled ethanol in the presence of sodium acetate (pH 5.2) and yeast tRNA. The DNA pellet was washed with 700 mL/L ethanol, air-dried, dissolved in 30 μL of TE (pH 8.0) and stored at 4 °C for further use. For isolation of cccDNA, the cells were washed and lysed as above. The lysate was collected in an eppendorf tube and 250 μL of 2.5 mol/L KCl was added and mixed properly. Following incubation on ice for 30 min, the mixture was centrifuged at 13 000 r/min for 30 min in a microfuge. The supernatant containing viral cccDNA was separated and subjected to phenol-chloroform-isoamyl alcohol extraction and ethanol precipitation, as above. The cccDNA pellet was dried, dissolved in 30 μL of TE and stored at 4 °C for further use.
Analysis of HBV DNA
HBV DNA isolated from Bac-HBV transduced cells, either untreated or treated with antiviral drugs, was analyzed by dot blot hybridization for total viral DNA or by Southern blotting for replicative intermediates according to standard protocols. An HBV full length (3.2 kb) linear DNA was radiolabeled with 32P dCTP, using a nick-translation kit (Invitrogen, Carlsbad, CA, USA) as per manufacturer’s instructions, purified through a Sephadex G50 column and used as a probe. The signals on dot blots and Southern blots were scanned, and the bands were quantitated by densitometry using the Kodak 1D image analysis software (Kodak Digital Science, Wauwatosa, WI, USA). The signal intensities were expressed as relative pixel values for each blot.
RESULTS
Generation and characterization of Bac-HBV
The subtype ayw infectious HBV clone pTKHH2 was used to isolate a 1.6 times genome-length HBV fragment as an XhoI-BglII fragment. This fragment included one complete copy each of the HBV P and S (including the pre-S regions) ORFs and two copies each of the C (including pre-C) and X ORFs (Figure 1A). The XhoI-BglII fragment was cloned into the baculovirus AcMNPV transfer vector pBacPak9 and a recombinant virus selected as described in Materials and Methods. Multiple recombinant baculoviruses were analyzed in three rounds of plaque purification. Since the HBV genome is placed in the transfer vector in an orientation opposite that of the baculovirus polyhedrin promoter, no HBV gene expression is expected in insect Sf21 cells used for recombinant baculovirus selection. However, in HepG2 cells the HBV transcription regulatory elements would be active and expression would be controlled by endogenous HBV promoters/enhancers. The Bac-HBV isolate A3-20 was finally selected for further experiments. The efficiency of transduction of HepG2 cells by Bac-HBV was estimated by HBsAg expression and total HBV DNA synthesis in transduced cells. Following transduction, we observed HBsAg synthesis (Figure 1B) and HBV DNA accumulation (Figure 1C) in HepG2 cells. High levels of HBsAg were observed in the culture medium starting at d 2 post-transduction and these levels remained constant till d 5 (Figure 1B). While it was not possible to carry on this HBsAg accumulation experiment beyond 5 d post-transduction, other experiments wherein the culture medium was changed every alternate day showed HBsAg expression to be uniformly high for up to 10 d post-transduction (data not shown). Total HBV DNA also accumulated in transduced HepG2 cells with a peak on d 6 followed by a rapid decline by d 10 (Figure 1C). The results showed that the Bac-HBV constructed here was functional and transduction of HepG2 cells led to the expression of HBV markers.
Effect of lamivudine and IFN-γ on HBV expression and replication
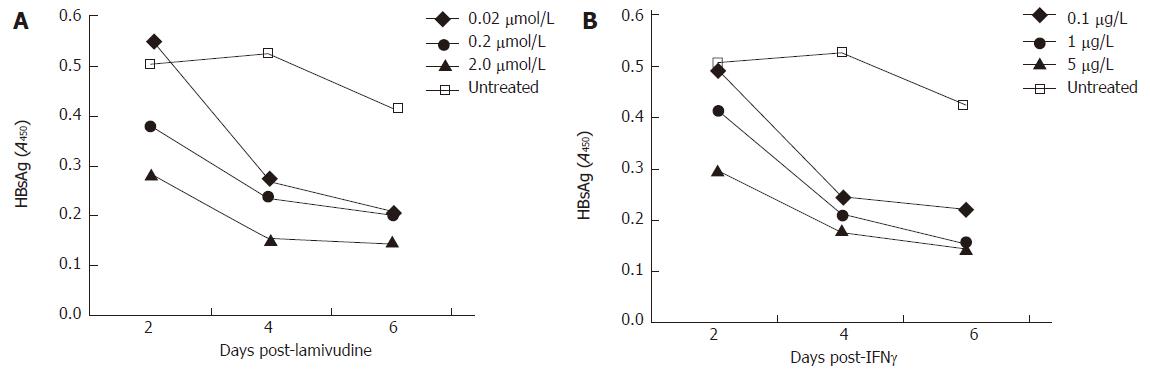
Since lamivudine is already established as a drug with anti-HBV properties, we tested its inhibition of the Bac-HBV system. In the range of 0.02 to 2 μmol/L lamivudine effectively reduced HBsAg levels in the culture medium of treated cells, compared to untreated cells (Figure 2A). A reproducible dose response was observed wherein with increasing drug concentration lower levels of HBsAg were observed at each of the three sampling points, d 2, 4 and 6 post-treatment. In the same experiment we also evaluated the anti-HBV effect of IFN-γ. At concentrations of 0.1 to 5 μg/L that corresponded to 3000 to 150 000 IU/L, IFN-γ also showed a marked reduction in HBsAg levels compared to untreated cells (Figure 2B). There was a rapid decline in HBsAg levels in the d 2 to d 4 period following IFN-γ treatment. We also tested whether cells pre-treated with IFN-γ and then transduced with Bac-HBV showed a similar response. HepG2 cells were either treated with IFN-γ for 24 h prior to transduction (pre-treatment group) or were first transduced and IFN-γ treatment was then initiated 24 h later (post-treatment group). In both cases, HBsAg levels in culture media were estimated 4 d after IFN-γ treatment. Pre-treatment with IFN-γ (0.01 to 10 μg/L) was found to have no significant effect on HBsAg expression, while treatment with IFN-γ after transduction was found to inhibit HBsAg expression, as observed earlier (data not shown).
Figure 2 A time course HBsAg profile (ELISA) of transduced HepG2 cells, treated with variable doses of lamivudine and IFN-γ.
A: Effect of lamivudine used at concentrations of 0.02, 0.2, and 2.0 μmol/L; B: Effect of IFN-γ used at concentrations of 0.1, 1.0 and 5.0 μg/L.
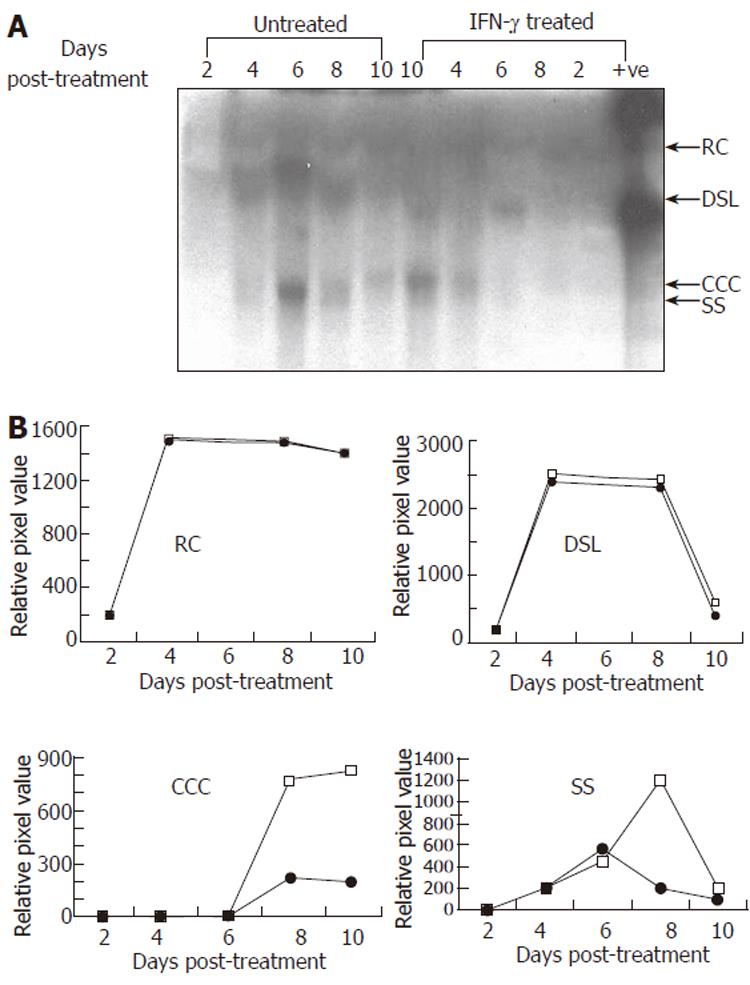
To evaluate the effect of IFN-γ on HBV replication, we transduced HepG2 cells with Bac-HBV followed by treatment with 5 μg/L IFN-γ as earlier, and extracted total DNA from untreated control and treated cells at regular intervals up to ten days following IFN-γ treatment. The replicative forms of HBV were separated, identified by Southern blotting and the signals quantitated by densitometry. The results are presented in Figure 3. While no significant effect of IFN-γ was observed on the relaxed circle (RC) and double-stranded linear (DSL) forms of HBV DNA, IFN-γ treatment led to significant reduction in the covalently closed circular (CCC) and single-stranded (SS) forms of HBV DNA. The latter forms appear late in infection and are indicative of viral DNA replication as seen from their appearance around d 6 post-treatment (or d 7 of Bac-HBV transduction). While the cccDNA remains high once replication has taken place, the ssDNA form is the replicative intermediate that increases transiently and disappears at late time points. Both these forms were suppressed around 75%-80% by IFN-γ. Overall, the kinetic study of HBV replication in transduced HepG2 cells demonstrated a time dependent, sequential synthesis of the different replicative intermediates and their inhibition by IFN-γ.
Figure 3 Representative Southern blot (A) and quantitative (densitometry) analysis (B) of effect of IFN-γ (5.
0 μg/L) treatment on HBV DNA replication in transduced HepG2 cells. Synthesis of viral replicative intermediates: relaxed coil (RC), double stranded linear (DSL), covalently closed circular (CCC), and single stranded (SS) DNA forms in treated (•) and untreated (□) cells.
Effect of combination of lamivudine and IFN-γ on HBV expression and replication
Our in vitro data on lamivudine and IFN-γ monotherapy showed the effective doses of the two agents to be 2.0 μmol/L and 5 μg/L, respectively scheduled for six days (Figure 2). Comparable antiviral effects were observed at these doses. We hypothesized that a combination of lower doses of these two agents would efficiently downregulate HBV gene expression and DNA replication. If this were the case, it would be significant in a clinical setting in reducing the time of lamivudine treatment and the possibility of developing drug resistance. To test this hypothesis, we designed an in vitro treatment schedule of Bac-HBV transduced HepG2 cells with mixed doses of lamivudine and IFN-γ for a period of six days.
We tested two groups, one with IFN-γ at 1 μg/L and 0.02 μmol/L, 0.2 μmol/L or 2 μmol/L lamivudine, and the other group with lamivudine at 0.2 μmol/L and 0.1 μg/L, 1 μg/L or 5 μg/L of IFN-γ. Following treatment of transduced cells with these combinations, HBsAg expression levels and HBV DNA load were estimated at d 2, 4 and 6 post-treatment. As a control in the same experiment, monotherapy with same concentrations of either lamivudine or IFN-γ was also evaluated. We observed no significant reduction in either HBsAg levels or HBV load for the combinations compared to the single drugs (data not shown).
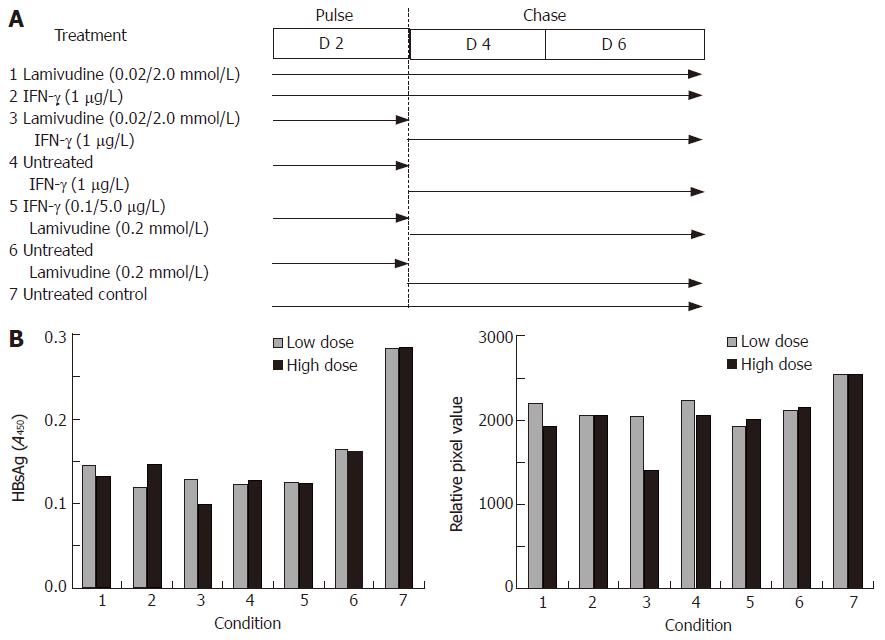
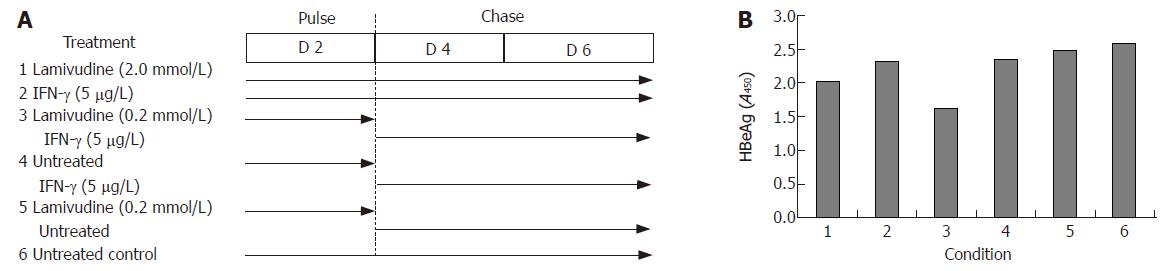
In view of these results, we further tested viral gene expression and DNA load using a sequential approach that included treatment with lamivudine alone for two days (pulse) followed by IFN-γ alone for the next four days (chase). We designed a “low” and “high” combination of the sequential treatment schedule for a period of 6 days (Figure 4A). No significant effect on either HBsAg expression (Figure 4B) or HBV DNA load (Figure 4C) was observed in the low dose sequential treatment group, when compared to either lamivudine or IFN-γ alone. However, in the high dose group, treatment first with 2 μmol/L lamivudine for 2 d followed by 1 μg/L IFN-γ for 4 d showed a significant and reproducible reduction in HBsAg expression and HBV DNA load compared to treatment with either agent alone. Since HBeAg expression and secretion in culture medium has been correlated with viral replication, we also tested its levels following lamivudine and IFN-γ treatment. Pulsing with 0.2 μmol/L lamivudine and chasing with 5 μg/L IFN-γ led to a significant reduction in HBeAg levels, compared to either agent alone (Figure 5).
Figure 4 Effects of sequential treatment with “low/high-dose” of lamivudine and IFN-γ on HBV gene expression and total DNA load in transduced HepG2 cells.
A: HBsAg profile; B: total HBV DNA upon sequential treatment of transduced cells. 1: Lamivudine (0.02 or 2.0 μmol/L) 6 d; 2: IFN-γ (1.0 μg/L) 6 d; 3: Lamivudine (0.02 or 2.0 μmol/L) 2 d + IFN-γ (1 μg/L) 4 d; 4: Untreated 2 d + IFN-γ (1.0 μg/L) 4 d; 5: IFN-γ (0.1 or 5 μg/L) 2 d + lamivudine (0.2 μmol/L) 4 d; 6: Untreated 2 d + lamivudine (0.2 μmol/L) 4 d; 7: Untreated control.
Figure 5 Effects of sequential treatment with “high-dose” of lamivudine and IFN-γ (A) on HBeAg expression in transduced HepG2 cells (B).
1: Lamivudine (2.0 μmol/L) 6 d; 2: IFN-γ (5.0 μg/L) 6 d; 3: Lamivudine (0.2 μmol/L) 2 d + IFN-γ (5 μg/L) 4 d; 4: Untreated 2 d + IFN-γ (5 μg/L) 4 d; 5: Lamivudine (0.2 μmol/L) 2 d and untreated 4 d; 6: Untreated control.
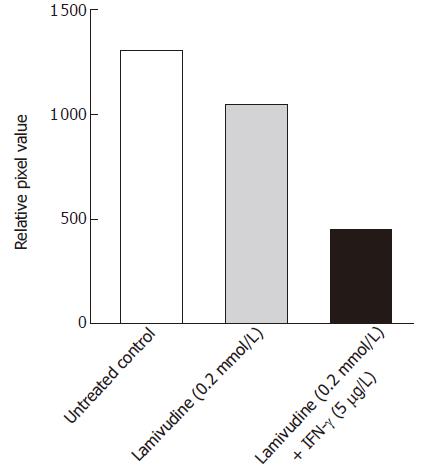
With this information, we extended our studies to evaluate the inhibitory effects of a 2 d pulse of 0.2 μmol/L lamivudine, followed by a 6 d chase with 5 μg/L IFN-γ on the cccDNA pool. Southern hybridization analysis of the HBV cccDNA, isolated from the nuclei of treated or untreated Bac-HBV transduced HepG2 cells showed about 60% suppression of cccDNA by this sequential dosing, compared to lamivudine alone (Figure 6). Compared to the untreated control, this sequential dose reduced cccDNA levels by about 72%; under similar conditions, lamivudine alone reduced cccDNA forms by about 30%. Based on our in vitro studies, a sequential therapy with lamivudine given as a pulse of 0.2 μmol/L for the first two days and chased with 5 μg/L IFN-γ for another six days was found to efficiently suppress HBV infection.
Figure 6 Effect of sequential treatment with “high-dose” of Lamivudine and IFN-γon HBV cccDNA pool in transduced HepG2 cells.
The figure shows densitometric analysis of the 2 kb cccDNA form. 1: Untreated control; 2: Lamivudine (0.2 μmol/L) 8 d; 3: Lamivudine (0.2 μmol/L) 2 d + IFN-γ (5.0 μg/L) 6 d.
DISCUSSION
A major hurdle in the development of new anti-HBV agents is the lack of suitable in vitro as well as in vivo systems, which could accurately or closely model chronic HBV infection in humans. Since HBV does not directly infect cells in culture, several physicochemical and virus vector-based methods have been used to deliver HBV genome to establish productive infection in vitro[25,26]. A recombinant baculovirus-based system has been used to transfer HBV genome into cultured liver cell lines[23] and to test the efficacy of antiviral agents[27]. There are multiple features that make this recombinant baculo-HBV/HepG2 culture system a useful tool over stable HBV-expressing cell lines such as HepG2.2.15[23]. It is a transient system that does not require integration of the HBV genome into the cellular DNA and thus more accurately mimics CHB in humans[23]. The HBV-baculovirus infection, even at high multiplicity of infection is not toxic to HepG2 cells, and HBsAg as well as HBV virions can be readily detected in the culture medium as early as 2-4 d post-infection. Many aspects of HBV biology, its pathogenicity and efficacies of antiviral drug have been studied in related hepadnaviruses like WHBV[28] and duck hepatitis B virus (DHBV)[29] that are useful models, but still different in the details of viral biology compared to HBV.
We developed our own Bac-HBV system based on published guidelines[23] and characterized it for HBsAg expression and the kinetics of HBV DNA accumulation. The analysis of HBV replicative intermediates in transduced HepG2 cells demonstrated a time-dependent, sequential synthesis of different viral DNA replicative intermediate species. The presence of high levels of dslDNA on the fourth day could be related to the onset of viral DNA replication, which coincided with the appearance of ssDNA (a hallmark of HBV replication) and kept increasing on the following days. A sudden appearance of high levels of cccDNA forms on the sixth day is likely to result from its accumulating pool in the nuclei of HepG2 cells. The cccDNA is the only transcription template for all HBV RNA species, and is maintained as minichromosomes complexed with nuclear histone proteins[24,30]. This further stabilizes this minichromosomal DNA, which in turn becomes resistant to nucleases[30,31]. The cccDNA also gets recycled from the cytoplasmic-nascent rcDNAs[24]. That is probably why we observed a high cccDNA pool throughout, once it reached its equilibrium on the eighth day. The ssDNA seen at its maximum on d 8, indicated that viral genome replication was at its peak at that time. A sudden drop in its level by d 10 showed cessation of replication. Here, it is important to note that like ssDNA, the dslDNA also dropped on d 10 from its d 8 peak levels. The dslDNA is a byproduct of a minor HBV DNA replication strategy called illegitimate replication, already shown in DHBV models[31]. In this pathway, dslDNA is produced as a result of the failure to prime plus-strand DNA synthesis at the correct location, and is efficiently converted to cccDNA by non-homologous recombination near the two ends of the linear DNA. It is speculated that the low levels of virus expression in cells carrying out illegitimate replication might provide such cells a survival advantage during an antigen-specific cellular immune response[32].
Compared to IFN-α, IFN-γ is a potent immuno-stimulatory agent and is the major cytokine responsible for regulating the expression of both class I and class II antigens[33]. The classical concept that viral clearance is mediated by CTLs, which may destroy the infected hepatocytes has been challenged by recent studies that virus specific CTLs can abolish HBV gene expression, transcription and replication without killing the infected hepatocytes[34]. However, the distinction between the antiviral and cytotoxic effects of IFN-γ on HBV infected hepatocytes is not well characterized. In our in vitro study, IFN-γ was found to be effective in down regulating HBsAg levels at concentrations of 1.0 and 5.0 μg/L. The higher dose of IFN-γ was effective in suppressing viral replication by altering the kinetics of replicative DNA intermediates. It was effective on ssDNA only after the sixth day of treatment and the levels were reduced to 50% on the following days, clearly indicating cessation of replication. Interestingly, the cccDNA pool that was not effectively eliminated by lamivudine, was suppressed by 75%-80% by IFN-γ on the very day it appeared. This might be due to destabilization and degradation of cccDNA under high dose IFN-γ pressure. However, IFN-γ failed to suppress the rcDNA and dslDNA forms, despite continued therapy.
The three aims of any therapeutic strategy for CHB are (1) to decrease viral load to undetectable levels, (2) to decrease the rate of disease progression and, (3) to decrease the rate of emergence of drug-resistant HBV. Combination therapy has emerged as a new approach to treat CHB wherein the objective is to first bring down the viral load to the lowest possible levels with a potent nucleoside drug. This is then followed by a continued chemotherapy with another nucleoside analog or IFN-α in order to maintain or eliminate the remaining viral load[35-37]. Continuation with another nucleoside may again give rise to emergence of novel drug-resistance. Following the same strategy, we also evaluated combinations of lamivudine and IFN-γ in our in vitro system. This novel approach effectively reduced the cccDNA pool from the nuclei of transduced HepG2 cells.
Only about a third of individuals exposed to HBV are clinically diagnosed with symptomatic acute hepatitis[38]. This infection is identified only sporadically, usually post-factum, by accidental detection of circulating viral markers and serum HBV DNA. However, it has been shown that clinical recovery with biochemical normalization of liver function, the clearance of serum HBsAg and HBV DNA and the appearance of anti-HBs do not reflect virus eradication after an episode of hepatitis B[38-40]. HBV genomes persist in serum and liver despite the clearance of serum HBsAg and anti-HBs seroconversion during antiviral therapy for CHB[41,42]. Consistent molecular evidence of WHV replication not only in the livers but also in peripheral blood mononuclear cell (PBMC), bone marrow, spleen, lymph nodes, and, occasionally, in the thymus also supports the findings in the HBV-human situation. Overall, the results from recent studies unequivocally demonstrate that low-rate hepadnavirus replication in serologically undetectable infection progresses in lymphoid cells[43,44]. The lymphoid cells are believed to be an incessant, non-hepatocyte reservoir of the viral cccDNA pool as a result of “occult” infection. It is likely that this silent cccDNA may reactivate synthesis of viral transcripts and proteins and therefore a rebound of active viral replication. Therefore, elimination of cccDNA pool from infected cells still remains a challenge in HBV therapy. To the best of our knowledge, no drug or combinations of drugs to date, has been shown to effectively eliminate cccDNA.
In this study, using a combination of lamivudine and IFN-γ, we were able to successfully eliminate about three-fourths of the HBV cccDNA pool from transduced HepG2 cells. In our dot-blot analysis of the total HBV DNA levels, there was a little reduction in treated cells compared to untreated controls. This can be explained by the kinetics of HBV DNA replicative intermediates, discussed above. IFN-γ was only found to be capable of inhibiting ssDNA and cccDNA molecules. The ssDNA levels peaked on the eighth day and effects of IFN-γ were seen afterwards. The accumulation of cccDNA also started after the sixth day. Therefore at day 6 post-transduction, we cannot expect cccDNA in the total HBV DNA pool. On the other hand, IFN-γ had no effect on the other two forms, rcDNA and dslDNA. So, it is only the ssDNA, which could be suppressed on the sixth day of treatment. This is why the levels of total HBV DNA were found to be slightly down regulated, compared to the untreated controls. Overall, our in vitro observations of inhibitory effects of IFN-γ on HBV gene expression and DNA replication are in agreement with the published data from other groups[13,15,16]. The differences between such studies however, could be related to different dose designs, duration of chemotherapy and method of preparation of the IFN-γ used.
In conclusion, we have used the HBV-baculovirus/HepG2 system to show that sequential treatment with lamivudine and IFN-γ efficiently suppressed HBV infection by down regulating viral replicative intermediates, particularly the cccDNA pool. These in vitro results support further clinical evaluation and inclusion of IFN-γ with lamivudine as an effective combination therapy for chronic HBV infection. Such an approach may benefit those patients who do not respond to IFN-α. Further, a shortened time-course of lamivudine treatment could also help minimize emergence of drug-resistant mutants of HBV in clinical situations.