Published online May 21, 2006. doi: 10.3748/wjg.v12.i19.2979
Revised: October 20, 2005
Accepted: October 26, 2005
Published online: May 21, 2006
Gastric cancer remains a global killer with a shifting burden from the developed to the developing world. The cancer develops along a multistage process that is defined by distinct histological and pathophysiological phases. Several genetic and epigenetic alterations mediate the transition from one stage to another and these include mutations in oncogenes, tumour suppressor genes and cell cycle and mismatch repair genes. The most significant advance in the fight against gastric caner came with the recognition of the role of Helicobacter pylori (H pylori) as the most important acquired aetiological agent for this cancer. Recent work has focussed on elucidating the complex host/microbial interactions that underlie the neoplastic process. There is now considerable insight into the pathogenesis of this cancer and the prospect of preventing and eradicating the disease has become a reality. Perhaps more importantly, the study of H pylori-induced gastric carcinogenesis offers a paradigm for understanding more complex human cancers. In this review, we examine the molecular and cellular events that underlie H pylori-induced gastric cancer.
- Citation: Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol 2006; 12(19): 2979-2990
- URL: https://www.wjgnet.com/1007-9327/full/v12/i19/2979.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i19.2979
Gastric cancer remains a major health problem being the fourth commonest cause of cancer death in Europe[1]. On a global scale, gastric cancer remains the world’s second commonest malignancy, having only been overtaken by lung cancer in the late 1980’s[2-4]. There is substantial international variation in gastric cancer incidence with the highest rates reported from Korea, Japan and eastern Asia. Other high incidence areas include Eastern Europe and parts of Latin America, while Western Europe and the US generally have low incidence rates. The global burden of gastric cancer is shifting rapidly from the developed world to the developing world. Despite the worldwide decline in incidence and the major improvements in diagnosis and treatment, less than 20% of patients survive to 5 years.
The vast majority of gastric cancers are sporadic. However there is strong evidence that occasional cases have an inherited component. Over 95% of malignancies of the stomach are adenocarcinomas. Lymphoma, sarcomas and carcinoid tumours comprise the remaining less common neoplasm’s. Adenocarcinoma of the stomach comprises a spectrum of different conditions classified according to the site of tumour origin and the pathological appearance of the lesion. Lauren composed a histological classification that is widely applied. According to this classification neoplasms are described as intestinal or diffuse types[5]. Intestinal tumours are comprised of malignant cells that are united to form structures resembling functional glands of the gastrointestinal tract. In contrast, the less common diffuse tumour type comprises cells that lack cohesion and are no longer capable of gastric function. The evolution of intestinal tumours has been characterised as progressing through a number of sequential steps. These steps begin with gastritis which progresses to mucosal atrophy (atrophic gastritis) followed by intestinal metaplasia, dysplasia and carcinoma with subsequent metastatic dissemination. No preceding steps have been identified in the pathogenesis of diffuse carcinoma other than the obvious chronic gastritis that is the hallmark of Helicobacter pylori (H pylori) pathogenesis. Diffuse adenocarcinoma has an increased propensity for intra and transmural spread and is therefore associated with a poorer prognosis. Unfortunately the histological classification of an individual gastric adenocarcinoma is not clear-cut with a tumour often comprising a mixture of intestinal and diffuse tissue types.
The pathogenesis of gastric cancer represents a classic example of gene-environment interactions[6,7]. For many decades it was well established that the cancer developed along well-defined histological and pathophysiological stages. Chronic inflammation with gastric atrophy was shown to be the most important pathological entity with hypochlorhydria being the most important physiological abnormality. Diets high in food preservatives such as salts and nitrates are thought to induce gastric malignancy whereas increased consumption of food containing natural antioxidants such as fresh fruit and vegetables may slow or prevent the disease. Alcohol and smoking are also thought to contribute to the aetiology. Achlorhydria, pernicious anaemia and blood group A are also associated with a higher risk of gastric malignancy.
Hereditary factors clearly increase the risk of gastric cancer and this malignancy is part of a number of familial cancer syndromes. The most celebrated familial case of gastric cancer is that of Napoleon Bonaparte. It is well documented at autopsy that the exiled Emperor had a malignant gastric ulcer complicated by a chronic perforation and haemorrhage. His father, Charles Bonaparte, died from scirrhous carcinoma of the pylorus at the age of 39, and his grandfather, Joseph Bonaparte, also died of “suspected” gastric cancer at the age of 40. At least one of his brothers and one of his sisters also died of the same malignancy[8]. Lending support to the genetic aetiological hypothesis is the recognition that patients with hereditary nonpolyposis colon cancer and Familial Adenomatous Polyposis are at an increased risk of developing malignancy in the stomach. Following the discovery of H pylori it is has become apparent that the preceding aetiological factors only account for a small proportion of the cases and this review will focus primarily on H pylori-induced sporadic gastric cancers.
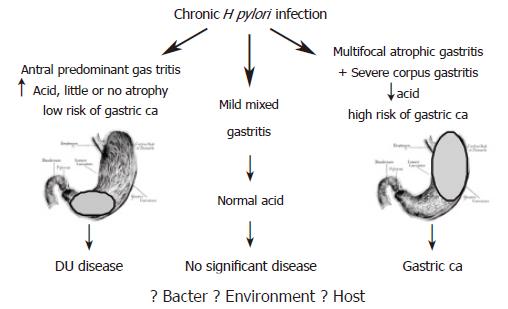
The discovery of H pylori infection in the early eighties proved a turning point in understanding the pathogenesis of gastric cancer[9]. While the link between H pylori and peptic ulcer disease was established soon after successful culture of the bacterium, the association with gastric cancer lagged almost a decade before credible evidence was presented. The major reason for this delay was inability to demonstrate the presence of active infection in gastric tissue of cancer patients. A major advance in this field came with the recognition that chronic H pylori infection induces physiological and morphological changes within the gastric milieu that increase the risk of neoplastic transformation. It is widely accepted that chronic H pylori infection induces hypochlorhydria and gastric atrophy, both of which are precursors of gastric cancer (Figure 1). Presence of the infection in the final stages of this cascade is therefore not necessary for cancer to develop as irreversible damage had already occurred.
The Helicobacter genus consists of at least 24 species found in the GI tracts of animals and humans. One of these species is H pylori a Gram-negative, spiral shaped, microaerophilic bacilli known to chronically infect over half the world’s population[10]. It is usually acquired in childhood, and if left untreated can persist for decades within the extreme environment of the human stomach[11]. The infection can be acquired via the faecal/oral or gastric/oral routes, and if not treated with antibiotics, can persist throughout life. The organism is non-invasive, nonspore-forming, measuring approximately 3.5 × 0.5 micrometers, with 4 to 6 unipolar flagellae[12]. These flagellae are protected from damage by the acidic environment of the stomach by a sheath. This allows the bacterium to move through the mucus layer within the stomach and to reside between this layer and the gastric epithelium[13]. Eighty percent of the bacilli are free-living, however the remainder adhere tightly to the underlying cells and induce ultra-structural changes in the gastric epithelial cell[10,14].
While H pylori is well equipped to colonise the inhospitable acidic gastric environment, it is essentially a neutralophile that grows best at a pH of between 6.0 and 8.0[13]. In order to do this, H pylori is equipped with several factors that allow it to colonise and evade the host defences including the immune response. H pylori possesses a urease enzyme that allows it to hydrolyse gastric urea into ammonia and carbon dioxide. This permits H pylori to maintain a constant internal and periplasmic pH, even in the presence of a very high external H+ concentration. In addition, H pylori expresses a urea transport protein (UreI) with unique acid-dependent properties that attenuates the rate of urea entry into the cytoplasm[15]. The combination of a neutral pH-optimum urease and an acid-regulated urea channel explains why H pylori is unique in its ability to inhabit the human stomach[16,17]. Indeed, isogenic urease-negative mutants of H pylori are incapable of colonising the gastric mucosa[18]. To conserve energy and resources, H pylori seeks out a niche that does not constantly challenge its acid-resisting and acid adaptation machinery. This explains why the initial colonisation is maximal in the antral part of the stomach, a region with a higher pH than the acid-producing corpus mucosa. This also explains why the distribution of infection changes when gastric acid secretion is inhibited by pharmacological means. In these circumstances, H pylori and its associated inflammation spread to involve the hitherto protected corpus mucosa.
Since early in the 20th century, it has been known that different patterns of gastritis can occur following H pylori infection, which ultimately results in differing clinical outcomes. The majority of H pylori infected individuals develop mild pangastritis, a condition that does not adversely alter gastric physiology and is not associated with significant disease (Figure 1). Antral predominant gastritis is associated with hyperchlorhydria, which carries a low risk of developing gastric cancer, but a high risk of developing duodenal ulcer disease[19]. In contrast corpus predominant gastritis, which leads to hypochlorhydria and gastric atrophy, carries an increased risk of gastric cancer[20]. It is thought that following the development of the hypochlorhydric atrophic gastric environment, other bacteria, such as nitrogen-fixing bacteria are able to colonise. These bacteria produce carcinogenic N-nitroso compounds through the conversion of nitrates, and the increasing mutagenic and genotoxic pressure coupled with the lack of free radical scavengers is thought to drive gastric cancer progression.
The key question is how chronic H pylori infection can be associated with such divergent clinical outcomes Much research has focussed on H pylori strain differences such as virulence factors as the source of the disease specificity. However, although these factors undoubtedly contribute to the severity of the disease, they do not define the clinical outcome[21]. This has prompted research in to other factors that could affect an individual’s response to H pylori infection. These factors include environmental and host genetic factors.
There is substantial evidence that genetic differences play a role in the clinical outcome of H pylori infection, particularly H pylori-virulence associated genes such as cagA, vacA, iceA and babA.
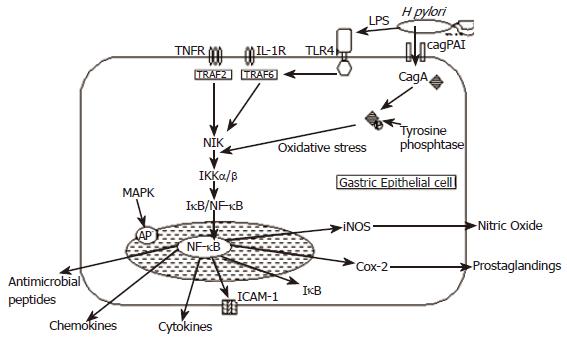
The cag Island: The best characterised H pylori virulence factor is the cag pathogenicity island (cag-PAI), a 40 kb chromosomal DNA, which contains approximately 31 genes[22,23]. Several of the genes present on the cag-PAI encode components of a type IV secretion system, which allows CagA (cytotoxin-associated gene A), a 120-130 kDa protein product and other bacterial proteins encoded by the cag-PAI to be injected into the epithelial cell cytosol (Figure 2)[10,12]. After entering the cell, CagA is phosphorylated and binds to tyrosine phosphatase, which induces secretion of IL-8, a potent chemotactic and activating factor for neutrophils, by the activation of nuclear factor kappa B (NF- κB) complexes[10,24]. The cag-PAI also induces cell surface remodelling including the induction of pedestal formation, activation of the transcription factor AP-1 and expression of the proto-oncogenes c-fos and c-jun by activation of the ERK/MAP kinase cascade[25,26]. H pylori strains which do not contain the cag-PAI or possess mutated cag genes do not induce these changes or do so to a much lesser extent[24,26,27].
H pylori strains can be divided into 2 groups based on the presence/absence of the cag-PAI: - type 1 strains, which possess the cag-PAI, and type 2 which do not. Type 1 strains are associated with severe gastritis, peptic ulcer disease, gastric atrophy and non-cardia gastric cancer, thus linking the presence of PAI to increased virulence[28-30]. It should be noted however, that not all type 1 isolates contain the entire PAI. Infection with a cagA expressing strain is also associated with reduced apoptosis whereas infection with a cagA negative strain is associated with increased apoptosis. Therefore cagA may act to inhibit gastric epithelial-programmed cell death.
vacA gene: The vacA gene encodes the expression of a vacuolating cytotoxin VacA, which induces vacuole formation in eukaryotic cells and stimulates epithelial-cell apoptosis[31,32]. The toxin inserts itself into the epithelial-cell membrane forming a voltage dependent channel through which bicarbonate and organic anions can be released. Unlike the cag-PAI, all H pylori strains possess the vacA gene, although only approximately 50% of strains express the VacA protein. Differences in expression are due to gene sequence variation[33]. Humans infected with VacA expressing H pylori demonstrate a greater degree of gastritis than non-expressing strains. H pylori infection is invariably associated with elevated gastric epithelial cell proliferation, thought to be a consequence of the epithelial damage.
babA gene: The babA gene encodes an outer-membrane protein BabA, which binds to fucosylated Lewis B blood group antigen on gastric cells[11,34]. BabA expressing strains adhere more tightly to gastric epithelial cells, and there is significant evidence accumulating that BabA expression may influence disease severity[11]. H pylori strains that possess babA, vacA and cagA carry the highest risk of gastric cancer[11].
iceA gene: A further putative virulence factor has descri-bed-iceA (induced by contact with epithelium) comprises two main variants iceA1 and iceA2[35]. However, the function of iceA2 is currently undefined[36]. Significant homology has been found between iceA1 gene and nlaIII a type II restriction endonuclease of Neisseria lactamica. Expression of iceA1 is up-regulated by contact of H pylori with human gastric epithelial cells and in some populations it is associated with peptic ulcer disease.
It is now well recognised that the development of gastric cancer and the precursory changes of gastric atrophy and hypochlorhydria are strongly influenced by host genetic factors. The importance of these factors was indicated by the association of Interleukin-1β gene polymorphisms and those affecting its receptor antagonist with an increased risk of developing hypochlorhydria and gastric atrophy in a Caucasian population of gastric cancer relatives, in the presence of H pylori[37]. The risk of gastric cancer is also exacerbated by carriage of these polymorphisms. The estimated odds ratio in a logistic regression model for IL-1B-511T +/-31C+ and IL-1RN*2/*2 were 1.6 (95% CI, 1.2-2.2) and 2.9 (95% CI, 1.9-4.4) respectively, in a Caucasian population[38]. The influence of these proinflammatory IL-1β and IL-1 receptor antagonist gene polymorphisms on the development of gastric pre-malignant lesions and carcinoma has subsequently been verified by other investigators[39,40]. IL-1β is relevant to the pathogenesis of H pylori-induced inflammation and the subsequent neoplastic process as the expression is up-regulated by the infection, it has proinflammatory actions and it is a powerful inhibitor of acid secretion[41]. Emphasising the multi-factorial nature of H pylori related gastric carcinoma, when these polymorphisms are combined with bacterial virulence factors, such as CagA, vacA s1 and vacA m1 positivity, the risk of developing the disease is greatly enhanced[36].
Several other cytokines in addition to IL-1β are involved in the inflammatory response to H pylori and are potential host-genetic risk factors. TNF-α is also up-regulated at an early stage after infection and subsequently influences transcription of several mediators[42]. Conversely IL-10 is an anti-inflammatory cytokine that inhibits cell-mediated immune responses. Functional, pro-inflammatory polymorphisms affecting both the genes encoding TNF-α and IL-10 have been associated with an increased risk of non-cardia gastric cancer[43]. The combination of three or four pro-inflammatory cytokine polymorphisms affecting IL-1β (IL-1B-511T), IL-1 receptor antagonist (IL-1RN*2), TNF-α (TNF-A-308A) and IL-10 (IL-10 haplotype ATA) results in a highly significant increased risk of developing non-cardia gastric cancer (OR 27.3 95% CI 7.4-99.8)[43]. More recently a functional pro-inflammatory polymorphism affecting the IL-8 gene (IL-8-251A) has been associated with increased gastric cancer[44-46].
Several other candidate genes have been studied in the attempt to discover associations with the development of gastric cancer. Cytochrome P450 is involved in the metabolism of dietary carcinogens such as N-nitrosamines and the CYP2E1 (c1/c1) genotype appears to be associated with an increased risk of cardia cancer, particularly in smokers[47]. HLA class II DR-DQ alleles also appear to influence gastric carcinoma development with DRB1*1601 being associated with an increased risk of gastric cancer, particularly in the absence of H pylori[48].
It is known that H pylori is a potent activator of NF-κB in gastric epithelial cells[49-52] (Figure 2). H pylori infection causes activation of the NF-κB pathway by a variety of mechanisms which are discussed in more detail below. Activation of NF-κB by H pylori induces nuclear translocation, which causes an increase in IL-8 messenger RNA and protein levels[50,53]. This has important implications since other NF-κB responsive genes including pro-inflammatory cytokines have been found in elevated levels in H pylori infected gastric mucosa. NF-κB activation is known to regulate cellular growth responses, including apoptosis, and is required for the induction of inflammatory and tissue-repair genes, including macrophage inflammatory protein (MIP)-2, metalloproteinase 3 (MMP3) and vascular endothelial growth factor (VEGF). The NF-κB pathway is also responsible for the generation of several cell adhesion molecules including ICAM-1 whose expression is significantly correlated with an increase in H pylori induced gastritis[54].
Lipopolysaccharide (LPS), which is a component of the outer membrane of Gram-negative bacteria including H pylori, is a signalling molecule for the innate immune system and is the main source of inflammation in Gram-negative infections[55]. LPS targets the transmembranous pattern-recognition receptor called toll-like receptor 4 (TLR4), which is expressed on macrophages and monocytes[56]. LPS binding to TLR4 activates signal transduction through MyD88, interleukin-1 receptor associated kinase and TRAF6 to activate the NF-κB and mitogen-activated protein kinase pathways[57-59], which leads to the synthesis and release of inflammatory cytokines such as IL-1, IL-8 and TNF-α , various chemokines GROα, IP10, MIG and MIP -1α & β[60], iNOS[61,62] and antimicrobial peptides providing a critical link to the adaptive immune system[63,64].
Induction of inflammation is an important component in the defence against H pylori. The presence of H pylori leads to the release of mutagenic substances such as metabolites of inducible nitric oxide synthase (iNOS), which is known to promote oncogenesis[65]. Nitric oxide generated by iNOS is converted to reactive nitrogen species, which can exert oncogenic effects including direct DNA and protein damage, inhibition of apoptosis, mutation of DNA and cellular repair functions such as p53 and also promotion of angiogenesis[66]. H pylori infection is also known to induce the expression of pro-inflammatory Cyclooxygenase enzyme (COX-2)[67-71]. COX-2 expression is normally undetectable in most normal tissues, but is induced rapidly during an inflammatory reaction[72,73]. Cox-2 activity is induced by a variety of mediators including inflammatory cytokines such as TNF-α, interferon-γ and IL-1[74]. COX-2 facilitates tumour growth by inhibiting apoptosis, maintaining cell proliferation and stimulating angiogenesis within cancer cells[75-77].
A positive association between ROS production and H pylori infective load has been established[78]. It has also been suggested that the source of ROS production was most probably host neutrophils, which had been activated by the presence of H pylori. It has also been shown that ROS production is enhanced by infection with cagA-positive H pylori strains[79].
The bacterial, environmental and host genetic factors discussed above influence the development of gastric carcinoma. In the following section, we discuss the molecular mechanisms underlying the disease. These include abnormalities of oncogenes, tumour suppressor genes, cell adhesion molecules and cell cycle regulators. Additionally genetic instability and alterations in growth factors and cytokines contribute to the complex pathways involved in gastric carcinogenesis. Differences exist in the pathways leading to diffuse- and intestinal-type gastric carcinoma, and these are summarised in Figures 3 and 4.
Many proto-oncogenes are activated in gastric carcinoma, with variations between the differing histological subtypes. The c-met gene, encoding a receptor for hepatocyte growth factor/scatter factor is amplified in 19% of intestinal-type and 39% of diffuse-type gastric cancers[80]. The majority of gastric carcinomas express two different c-met transcripts, of 7.0 kb and 6.0 kb. Expression of the 6.0kb transcript correlates well with prognostic factors such as tumour staging, depth of tumour invasion and lymph node metastasis[81]. The K-sam (KATO-III cell-derived stomach cancer amplified) oncogene is also frequently activated in gastric carcinomas, and has at least four transcriptional variants[82]. One of these, Type II, encodes a receptor for keratinocyte growth factor. K-sam is preferentially amplified in 33% of advanced diffuse or scirrhous -type gastric carcinomas but not in intestinal-type cancers[83]. Over-expression of this gene in gastric carcinoma is associated with a poorer prognosis.
Another proto-oncogene, c-erbB2, is preferentially amplified in 20% of intestinal-type gastric cancers but this is not a feature of the diffuse-type[84]. Over-expression of this gene is also correlated with poorer prognosis and liver metastases[85,86]. Mutations of K-ras are seen in intestinal-type gastric adenocarcinomas and the precursor lesions intestinal metaplasia and adenomas[87-89]. The incidence of this mutation is low and it is not a feature of diffuse-type carcinomas.
The tumour suppressor gene p53 is frequently inactivated in gastric carcinoma by loss of heterozygosity (LOH), missense mutations and frame shift deletions. This occurs in over 60% of gastric cancers, regardless of the histological subtype, and is frequently observed in precursor lesions such as intestinal metaplasia, dysplasia and adenomas[88,90-94]. Mutations commonly occur at A:T sites in intestinal-type carcinomas, with GC-AT transitions being common in diffuse-type carcinomas[91]. These GC-AT transitions can be caused by carcinogenic N-nitrosamines that are found in several foodstuffs and can be produced from dietary amines and nitrates in the acidic gastric environment[95,96]. Mutations in the codon 72 of exon 4 of the p53 gene have recently been associated with an increased risk of distal gastric cancer[97]. LOH of p73, a tumour suppressor gene related to p53 is detected in 38% of gastric cancers, and alterations of this gene are predominant features of foveolar-type gastric cancers with pS2 expression[98]. pS2 is a gastric-specific trefoil factor normally expressed in gastric foveolar epithelial cells. Inactivation of the pS2 gene results in dysplasia, adenoma and adenocarcinomas in mice[99,100]. Reduction or loss of the pS2 gene by DNA methylation in the promoter region occurs in intestinal metaplasia and gastric adenomas, suggesting this process may be important at an early stage in intestinal-type gastric carcinoma development[101].
Mutations of the tumour suppressor gene APC, involved in familial polyposis coli, are also observed in intestinal-type gastric carcinoma[102]. Although APC gene missense mutations are common in the intestinal subtype, occurring in over 50% of cases, they are not involved in diffuse-type cancers. Somatic mutations of the APC gene are observed in 20%-40% of gastric adenomas and 6% of intestinal metaplasias[103,104]. The expression of β-catenin, which acts as an oncogene, is enhanced by APC inactivation.
A further tumour suppressor is nuclear retinoic acid receptorβ (RARβ). Hypermethylation of this gene with reduced expression is observed in 64% of intestinal gastric cancers but this is not observed in the diffuse subtype[105]. Additional tumour suppressor gene alterations include those affecting distinct chromosomal loci. LOH at 1q and 7q are frequently associated with intestinal-type cancers while 1p is commonly affected in advanced diffuse cancers[88]. LOH of the bcl-2 gene is also frequently observed in intestinal-type cancers[106].
The RUNX gene family is composed of three members, RUNX1/AML1, RUNX2 and RUNX3[107]. It also encodes the DNA-binding α subunits of the Runt domain transcription factor polyomavirus enhancer-binding protein 2 (PEBP2)/core-binding factor (CBF), which is a heterodimeric transcription factor. Of the RUNX family, RUNX3 is involved in gastric carcinogenesis, being necessary for the suppression of cell proliferation in the gastric epithelium. The gastric epithelium of RUNX3 knockout mice exhibits hyperplasia, reduced rate of apoptosis and reduced sensitivity to TGFβ1, suggesting the tumour suppressor activity of RUNX3 operates downstream of the TGFβ signalling pathways. In humans, loss of RUNX3 by hypermethylation of the promoter CpG island is observed in several different cancers, including 64% of gastric carcinomas. RUNX3 methylation is also a feature of 8% of chronic gastritis, 28% of intestinal metaplasia and 27% of gastric adenomas[108]. This suggests RUNX3 is a target for epigenetic gene silencing in gastric carcinogenesis[109,110].
Other genes that appear to be affected in gastric carcinogenesis include the FHIT gene and loss of heterozygosity at the DCC locus, which is a feature of intestinal-type cancers[88,111]. Promoter hypomethylation of a novel cancer/testis antigen gene CAGE has recently been described in 35% of chronic gastritis and 78% of gastric cancer[112]. Histone H4 is progressively deacetylated during the development of gastric cancer, and this is a common event in both intestinal-type and diffuse type cancers[113].
Cell adhesion molecules may act as tumour suppressors, with mutations in the E-cadherin gene occurring preferentially in 50% of diffuse type gastric carcinoma[114]. This homophilic cell adhesion molecule belongs to a family of cell-cell adhesion molecules with an important role in intercellular adhesion by establishing cell polarity, maintaining tissue morphology and cellular differentiation in normal cells[115,116]. E-cadherin binds to the actin cytoskeleton via a series of catenin proteins[117]. Therefore changes in E-cadherin expression have a direct effect on cell adhesion and therefore plays an important step in cancer development. E-cadherin mutations affecting exons 8 or 9 induce the scattered morphology, decreased cellular adhesion and increased cellular motility of diffuse gastric cancers[118]. Mutations in β-catenin and γ-catenin have also been observed in gastric cancer cell lines, and together with E-cadherin mutations appear to be involved in the development and progression of diffuse and schirrhous-type cancers[119-121].
Abnormal CD44 transcripts are frequently associated with gastric carcinomas and metastatic deposits, with the pattern of these abnormal transcripts varying between the intestinal and diffuse subtypes[122]. All gastric cancer cell lines and tissues exhibit abnormal CD44 transcripts containing the intron 9 sequence[123]. This feature is also observed in 60% of gastric intestinal metaplasias but is absent from normal mucosa[124]. Osteopontin (OPN), a protein ligand of CD44, is overexpressed in 73% of gastric carcinomas and when co-expressed with CD44v9 correlates lymphatic invasion and metastasis[125,126]. Reduced expression of nm23, involved in c-myc transcriptional activation, and galectin-3, a galactoside-binding protein, are also implicated in metastatic gastric carcinoma[127,128].
The cell-cycle regulator, cyclin E, is amplified in 15%-20% of gastric carcinomas that are associated with its overexpression. Gene amplification or overexpression of cyclin E are associated with aggressiveness and lymph node metastasis[129]. The expression of the CDK inhibitor p27 that binds to a wide variety of cyclin/CDK complexes and inhibits kinase activity is frequently reduced in advanced gastric carcinoma while being preserved the majority of gastric adenomas and early cancers[130]. Reduced p27 expression correlates with tumour invasion and nodal metastasis. This reduction in p27 occurs at a post-translational level, and results not from genetic abnormalities but rather from ubiquitin-mediated proteosomal degradation[131]. A family of E2F transcription factors is an important target of cyclin/CDKs at the G1/s transition. Overexpression of E2F is observed in 40% of primary gastric cancers, and this tends to be co-expressed with cyclin E[132]. Gene amplification and abnormal expression of the E2F gene may permit the development of gastric cancer.
Microsatellite instability (MSI) is a hallmark of the DNA mismatch repair deficiency that is one of the pathways of gastric carcinogenesis. Microsatellites are short DNA sequence repeats that are scattered throughout the human genome and occur in nearly every case of gastric cancer associated with germline mutations of the mismatch repair (MMR) genes hMSH2, hMLH1, hPMS1, hPMS2, and MSH6/GTBP[133,134]. Errors that occur in DNA mismatch repair mechanisms in tumour cells can cause expansion and contraction of these repeats. MSI due to epigenetic inactivation of hMLH1 is found in 15%-39% of sporadic intestinal-type cancer, 70% of which are associated with loss of hMLH1 by hypermethylation of the promoter[135,136]. Such intestinal type cancers with MSI often occur in older patients and arise in the antrum. They are associated with lymphocyte infiltration, multiple tumours and a potentially favourable prognosis. Meanwhile MSI of the D1S191 locus is found in 26% of intestinal metaplasia and 46% of intestinal type gastric cancer. An identical pattern of this MSI of D1S191 is observed in adjacent intestinal metaplasia and intestinal type cancer that suggests the sequential development from the former to the latter[137]. Diffuse type cancers with MSI are more commonly observed in younger patients and have no germline mutations of hMLH1 and hMSH2, with no alteration in BAT-RII[138]. However, these cancers are frequently associated with LOH on chromosome 17q21 including the BRCA1 gene.
Human telomerase reverse transcriptase (hTERT) is an important determinant of telomerase activity, the enzyme that catalyses the telomere DNA synthesis. The majority of intestinal carcinomas have shortened telomere length, high levels of telomerase activity and a significant expression of hTERT[139]. Over 50% of intestinal metaplasias express low levels of telomerase activity, equivalent to 10% of the activity in gastric carcinoma[140]. hTERT is unregulated at an early stage in gastric carcinogenesis and H pylori may act as a trigger factor for hyperplasia in hTERT positive “stem cells” in intestinal metaplasia[139].
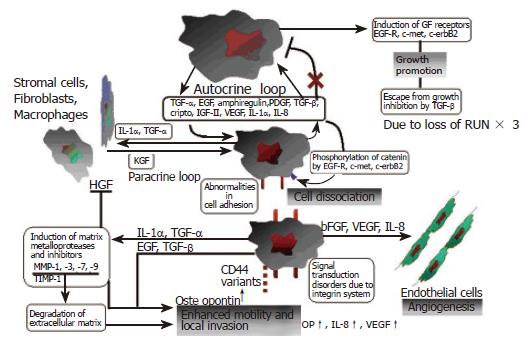
Gastric cancer cells express a wide array of growth factors and cytokines that act via autocrine, paracrine and juxtacrine mechanisms. Again the expression of these mediators varies depending on the histological subtype. These interactions are summarised in Figure 5. The EGF family, which includes EGF, TGFα, IGF II and bFGF, are commonly overexpressed in intestinal-type carcinoma. Meanwhile TGFβ, IGF II and bFGF are predominantly overexpressed in the diffuse subtype[101]. Co-expression of EGF/TGFα, EGFR and cripto correlates well with the biological malignancy, as these factors induce metalloproteinases[141,142]. Overexpression of cripto is frequently associated with intestinal metaplasia and gastric adenoma[143]. Gastric cancer cells express neutrophilin-1 (NRP-1), a co-receptor for VEGF receptor 2 endothelial cells[144]. EGF induces both NRP-1 and VEGF expression, suggesting that regulation of NRP-1 expression in gastric cancer is intimately associated with the EGF/EGFR system.
Interleukin-1α is produced by inflammatory cells and also gastric cancer cells. It acts as an autocrine growth factor for gastric carcinoma cells and is important in EGF and EGF receptor expression[145]. The interplay between IL-1α and the EGF/receptor system acts to stimulate gastric cancer growth. IL-6 also acts in an autocrine fashion to stimulate gastric cancer cells. Il-1α and IL-6 both stimulate the expression of each other by tumour cells{167}. IL-8, a member of the CXC family of chemokines has numerous roles in gastric carcinogenesis with over 80% of gastric tumours expressing both this cytokine and its receptor[146,147]. IL-8 enhances expression of EGF receptor, type IV collagenase (metalloproteinase (MMP)-9), VEGF and IL-8 mRNA itself by gastric cancer cells, while reducing E-cadherin mRNA expression.
The negative growth factor TGFβ is frequently overexpressed in gastric carcinoma, particularly diffuse type carcinomas with diffusely productive fibrosis[148]. Angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and IL-8 are produced by tumour cells and result in neovascularisation within gastric carcinoma tissue. VEGF promotes angiogenesis and progression of gastric carcinomas, particularly those of the intestinal subtype, while bFGF is has a stronger association for diffuse gastric carcinoma[149,150]. HGF/SF (hepatocyte growth factor/scatter factor) is produced by stimulated stromal cells such as fibroblasts, and functions in a paracrine manner as a morphogen or motogen[101].
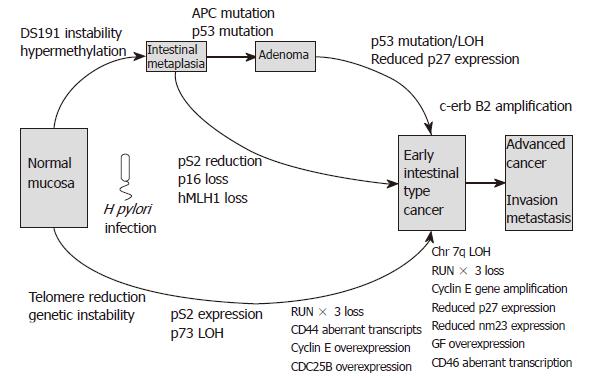
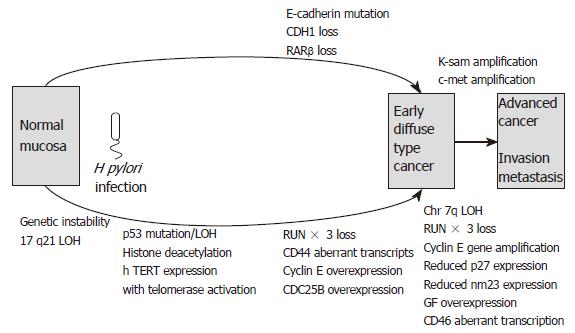
It can be seen that several of the molecular mecha-nisms are distinct for intestinal- and diffuse-type gastric carcinoma development, while some are common to both. Regarding intestinal-type carcinogenesis, there are three possible routes leading to carcinoma development. Firstly, progression through the pre-cancerous lesions of intestinal metaplasia to adenoma and finally carcinoma. Secondly, intestinal metaplasia may proceed directly to carcinoma. The third route involves the development of de novo gastric carcinoma with no preceding stage.
The first two pathways are summarised in Figure 3 and Figure 4. Genetic instability and hyperplasia of hTERT positive stem cells precede replication error at the DS191 locus, DNA hypermethylation at the D17S5 locus, pS2 loss, RARβ loss, RUNX3 loss, CD44 abnormal transcripts and p53 mutation, all of which accumulate in 30% of incomplete intestinal metaplasia. All of these epigenetic and genetic changes are common events in intestinal-type cancers.
An adenoma to carcinoma sequence is observed in around 20% of gastric adenomas with APC mutations. Molecular events associated with this sequence are loss of heterozygosity and mutation of p53, reduced p27 expression, loss of RUNX3, over-expression of cyclin E and abnormal c-met transcription. The resulting advanced intestinal-type gastric carcinomas frequently exhibit DCC loss, APC mutations, 1qLOH, loss of p27, reduced TGFβ receptor expression, reduced nm23 and c-erbB2 gene amplification. The “de novo” pathway of gastric carcinoma development involves LOH and abnormal expression of p73 exhibited in the development of foveolar-type gastric cancers. Meanwhile, diffuse-type gastric carcinogenesis involves LOH at chromosome 17p, MOH or mutation of p53, RUNX3 loss and mutation or loss of E-cadherin. Several of the above molecular events may be present in mixed gastric cancers that have both intestinal and diffuse components.
It is evident that gastric carcinoma results from a complex interaction between bacterial, environmental, host-genetic and molecular mechanisms. The importance of H pylori and the mechanisms by which the host recognises and responds to the bacterium are crucial in determining the resulting phenotype. Several common events are shared between the differing histological subtypes while distinct differences also highlight the fascinating divergence in histogenesis. The aetiology of these differences remains to be elucidated. Better understanding of these events will no doubt contribute significantly to our fight against this killer cancer. More importantly, the improved understanding of the pathogenesis of gastric cancer will serve as a paradigm for understanding other human cancers.
S- Editor Wang J E- Editor Ma WH
| 1. | Black RJ, Bray F, Ferlay J, Parkin DM. Cancer incidence and mortality in the European Union: cancer registry data and estimates of national incidence for 1990. Eur J Cancer. 1997;33:1075-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 327] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Correa P. Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiol Biomarkers Prev. 1996;5:477-481. [PubMed] |
| 3. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1710] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 4. | Kuniyasu H, Yasui W, Yokozaki H, Tahara E. Helicobacter pylori infection and carcinogenesis of the stomach. Langenbecks Arch Surg. 2000;385:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 6. | Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1344] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 7. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11281] [Article Influence: 490.5] [Reference Citation Analysis (2)] |
| 8. | WOOLF CM, ISAACSON EA. An analysis of 5 "stomach cancer families" in the state of Utah. Cancer. 1961;14:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 10. | Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 12. | Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Segal ED, Lange C, Covacci A, Tompkins LS, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci U S A. 1997;94:7595-7599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 15. | Suerbaum S. Genetic variability within Helicobacter pylori. Int J Med Microbiol. 2000;290:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Scott DR, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology. 1998;114:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 18. | Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604-3607. [PubMed] |
| 19. | Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, Fraumeni JF Jr, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 430] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3185] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 21. | Graham DY, Yamaoka Y. Disease-specific Helicobacter pylori virulence factors: the unfulfilled promise. Helicobacter. 2000;5 Suppl 1:S3-S9; discussion S27-S31. [PubMed] |
| 22. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2635] [Cited by in RCA: 2587] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 23. | Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1358] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 24. | Keates S, Keates AC, Warny M, Peek RM Jr, Murray PG, Kelly CP. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag- Helicobacter pylori. J Immunol. 1999;163:5552-5559. [PubMed] |
| 25. | Suerbaum S, Josenhans C, Claus H, Frosch M. Bacterial genomics: seven years on. Trends Microbiol. 2002;10:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 482] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl HL. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-kappaB activation. Infect Immun. 1998;66:2346-2348. [PubMed] |
| 28. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 29. | Cover TL, Dooley CP, Blaser MJ. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603-610. [PubMed] |
| 30. | Peek RM Jr, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760-770. [PubMed] |
| 31. | Peek RM Jr, Vaezi MF, Falk GW, Goldblum JR, Perez-Perez GI, Richter JE, Blaser MJ. Role of Helicobacter pylori cagA(+) strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int J Cancer. 1999;82:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080-5087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Peek RM Jr. Helicobacter pylori strain-specific modulation of gastric mucosal cellular turnover: implications for carcinogenesis. J Gastroenterol. 2002;37 Suppl 13:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 842] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 35. | Peek RM Jr, Thompson SA, Donahue JP, Tham KT, Atherton JC, Blaser MJ, Miller GG. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998;110:531-544. [PubMed] |
| 36. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, Williams C, Fullarton G, McColl KE. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 207] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1676] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 39. | Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, Gerhard M, Prinz C. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Genta RM. The immunobiology of Helicobacter pylori gastritis. Semin Gastrointest Dis. 1997;8:2-11. [PubMed] |
| 43. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 44. | Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, Koike T, Sekine H, Ohara S, Shimosegawa T. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Savage SA, Abnet CC, Haque K, Mark SD, Qiao YL, Dong ZW, Dawsey SM, Taylor PR, Chanock SJ. Polymorphisms in interleukin -2, -6, and -10 are not associated with gastric cardia or esophageal cancer in a high-risk chinese population. Cancer Epidemiol Biomarkers Prev. 2004;13:1547-1549. [PubMed] |
| 46. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Cai L, Zheng ZL, Zhang ZF. Cytochrome p450 2E1 polymorphisms and the risk of gastric cardia cancer. World J Gastroenterol. 2005;11:1867-1871. [PubMed] |
| 48. | Magnusson PKE H, Eriksson I, Held M, Nyrén O, Engstrand L, Hansson LE, Gyllensten UB. Gastric cancer and human leukocyte antigen: distinct DQ and DR alleles are associated with development of gastric cancer and infection by Helicobacter pylori. Cancer Res. 2001;61:2684-2689. [PubMed] |
| 49. | Mori N, Wada A, Hirayama T, Parks TP, Stratowa C, Yamamoto N. Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-kappaB in gastric epithelial cancer cells. Infect Immun. 2000;68:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 309] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 53. | Sharma SA, Tummuru MK, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401-2407. [PubMed] |
| 54. | Hatz RA, Rieder G, Stolte M, Bayerdörffer E, Meimarakis G, Schildberg FW, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 769] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 56. | Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 3805] [Article Influence: 135.9] [Reference Citation Analysis (0)] |
| 57. | Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-3752. [PubMed] |
| 58. | Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1580] [Cited by in RCA: 1572] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 59. | Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887-5894. [PubMed] |
| 60. | Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928-4937. [PubMed] |
| 61. | Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2442] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 62. | Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1606] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 63. | Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267-269. [PubMed] |
| 64. | Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697-4701. [PubMed] |
| 65. | Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238-3243. [PubMed] |
| 66. | Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626-G634. [PubMed] |
| 67. | Salvemini D, Seibert K, Masferrer JL, Settle SL, Misko TP, Currie MG, Needleman P. Nitric oxide and the cyclooxygenase pathway. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:491-493. [PubMed] |
| 68. | Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240-7244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 964] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 69. | Sawaoka H, Tsuji S, Tsujii M, Gunawan ES, Nakama A, Takei Y, Nagano K, Matsui H, Kawano S, Hori M. Expression of the cyclooxygenase-2 gene in gastric epithelium. J Clin Gastroenterol. 1997;25 Suppl 1:S105-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] |
| 71. | Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 477] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 73. | Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157-33160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 346] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 74. | Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063-1073. [PubMed] |
| 76. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 77. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 1565] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 78. | Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 303] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 79. | Suzuki H, Miura S, Imaeda H, Suzuki M, Han JY, Mori M, Fukumura D, Tsuchiya M, Ishii H. Enhanced levels of chemiluminescence and platelet activating factor in urease-positive gastric ulcers. Free Radic Biol Med. 1996;20:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun. 1992;189:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 241] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Kuniyasu H, Yasui W, Yokozaki H, Kitadai Y, Tahara E. Aberrant expression of c-met mRNA in human gastric carcinomas. Int J Cancer. 1993;55:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Katoh M, Hattori Y, Sasaki H, Tanaka M, Sugano K, Yazaki Y, Sugimura T, Terada M. K-sam gene encodes secreted as well as transmembrane receptor tyrosine kinase. Proc Natl Acad Sci U S A. 1992;89:2960-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Hattori Y, Odagiri H, Nakatani H, Miyagawa K, Naito K, Sakamoto H, Katoh O, Yoshida T, Sugimura T, Terada M. K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci U S A. 1990;87:5983-5987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283-287. [PubMed] |
| 85. | Oda N, Tsujino T, Tsuda T, Yoshida K, Nakayama H, Yasui W, Tahara E. DNA ploidy pattern and amplification of ERBB and ERBB2 genes in human gastric carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Yonemura Y, Ninomiya I, Ohoyama S, Kimura H, Yamaguchi A, Fushida S, Kosaka T, Miwa K, Miyazaki I, Endou Y. Expression of c-erbB-2 oncoprotein in gastric carcinoma. Immunoreactivity for c-erbB-2 protein is an independent indicator of poor short-term prognosis in patients with gastric carcinoma. Cancer. 1991;67:2914-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Lee KH, Lee JS, Suh C, Kim SW, Kim SB, Lee JH, Lee MS, Park MY, Sun HS, Kim SH. Clinicopathologic significance of the K-ras gene codon 12 point mutation in stomach cancer. An analysis of 140 cases. Cancer. 1995;75:2794-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Sano T, Tsujino T, Yoshida K, Nakayama H, Haruma K, Ito H, Nakamura Y, Kajiyama G, Tahara E. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res. 1991;51:2926-2931. [PubMed] |
| 89. | Isogaki J, Shinmura K, Yin W, Arai T, Koda K, Kimura T, Kino I, Sugimura H. Microsatellite instability and K-ras mutations in gastric adenomas, with reference to associated gastric cancers. Cancer Detect Prev. 1999;23:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Tamura G, Kihana T, Nomura K, Terada M, Sugimura T, Hirohashi S. Detection of frequent p53 gene mutations in primary gastric cancer by cell sorting and polymerase chain reaction single-strand conformation polymorphism analysis. Cancer Res. 1991;51:3056-3058. [PubMed] |
| 91. | Yokozaki H, Kuniyasu H, Kitadai Y, Nishimura K, Todo H, Ayhan A, Yasui W, Ito H, Tahara E. p53 point mutations in primary human gastric carcinomas. J Cancer Res Clin Oncol. 1992;119:67-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Tohdo H, Yokozaki H, Haruma K, Kajiyama G, Tahara E. p53 gene mutations in gastric adenomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Sakurai S, Sano T, Nakajima T. Clinicopathological and molecular biological studies of gastric adenomas with special reference to p53 abnormality. Pathol Int. 1995;45:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Ochiai A, Yamauchi Y, Hirohashi S. p53 mutations in the non-neoplastic mucosa of the human stomach showing intestinal metaplasia. Int J Cancer. 1996;69:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Sugimura T, Fujimura S, Baba T. Tumor production in the glandular stomach and alimentary tract of the rat by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1970;30:455-465. [PubMed] |
| 96. | Mirvish SS. Kinetics of nitrosamide formation from alkylureas, N-alkylurethans, and alkylguanidines: possible implications for the etiology of human gastric cancer. J Natl Cancer Inst. 1971;46:1183-1193. [PubMed] |
| 97. | Pérez-Pérez GI, Bosques-Padilla FJ, Crosatti ML, Tijerina-Menchaca R, Garza-González E. Role of p53 codon 72 polymorphism in the risk of development of distal gastric cancer. Scand J Gastroenterol. 2005;40:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Yokozaki H, Shitara Y, Fujimoto J, Hiyama T, Yasui W, Tahara E. Alterations of p73 preferentially occur in gastric adenocarcinomas with foveolar epithelial phenotype. Int J Cancer. 1999;83:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895-7903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 569] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 100. | Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, Wendling C, Tomasetto C, Chambon P, Rio MC. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 377] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 101. | Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;327-349. [PubMed] |
| 102. | Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1636] [Cited by in RCA: 1552] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 103. | Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y, Horii A. Somatic mutations of the APC gene in precancerous lesion of the stomach. Hum Mol Genet. 1993;2:1463-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, Horii A. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Hayashi K, Yokozaki H, Goodison S, Oue N, Suzuki T, Lotan R, Yasui W, Tahara E. Inactivation of retinoic acid receptor beta by promoter CpG hypermethylation in gastric cancer. Differentiation. 2001;68:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Ayhan A, Yasui W, Yokozaki H, Seto M, Ueda R, Tahara E. Loss of heterozygosity at the bcl-2 gene locus and expression of bcl-2 in human gastric and colorectal carcinomas. Jpn J Cancer Res. 1994;85:584-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Ito Y. Oncogenic potential of the RUNX gene family: 'overview'. Oncogene. 2004;23:4198-4208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 108. | Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW, Bang YJ, Kang GH. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004;84:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Sakakura C, Hagiwara A, Miyagawa K, Nakashima S, Yoshikawa T, Kin S, Nakase Y, Ito K, Yamagishi H, Yazumi S. Frequent downregulation of the runt domain transcription factors RUNX1, RUNX3 and their cofactor CBFB in gastric cancer. Int J Cancer. 2005;113:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 835] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 111. | Tamura G, Sakata K, Nishizuka S, Maesawa C, Suzuki Y, Iwaya T, Terashima M, Saito K, Satodate R. Analysis of the fragile histidine triad gene in primary gastric carcinomas and gastric carcinoma cell lines. Genes Chromosomes Cancer. 1997;20:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 112. | Cho B, Lee H, Jeong S, Bang YJ, Lee HJ, Hwang KS, Kim HY, Lee YS, Kang GH, Jeoung DI. Promoter hypomethylation of a novel cancer/testis antigen gene CAGE is correlated with its aberrant expression and is seen in premalignant stage of gastric carcinoma. Biochem Biophys Res Commun. 2003;307:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Ono S, Oue N, Kuniyasu H, Suzuki T, Ito R, Matsusaki K, Ishikawa T, Tahara E, Yasui W. Acetylated histone H4 is reduced in human gastric adenomas and carcinomas. J Exp Clin Cancer Res. 2002;21:377-382. [PubMed] |
| 114. | Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845-3852. [PubMed] |
| 115. | Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 327] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 116. | Smith ME, Pignatelli M. The molecular histology of neoplasia: the role of the cadherin/catenin complex. Histopathology. 1997;31:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Tucker EL, Pignatelli M. Catenins and their associated proteins in colorectal cancer. Histol Histopathol. 2000;15:251-260. [PubMed] |
| 118. | Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Höfler H. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene. 1999;18:4301-4312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 119. | Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol. 1995;15:1175-1181. [PubMed] |
| 120. | Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon ER. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 1999;10:369-376. [PubMed] |
| 121. | Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S. Dominant negative inhibition of the association between beta-catenin and c-erbB-2 by N-terminally deleted beta-catenin suppresses the invasion and metastasis of cancer cells. Oncogene. 1996;13:883-889. [PubMed] |
| 122. | Yokozaki H, Ito R, Nakayama H, Kuniyasu H, Taniyama K, Tahara E. Expression of CD44 abnormal transcripts in human gastric carcinomas. Cancer Lett. 1994;83:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Higashikawa K, Yokozaki H, Ue T, Taniyama K, Ishikawa T, Tarin D, Tahara E. Evaluation of CD44 transcription variants in human digestive tract carcinomas and normal tissues. Int J Cancer. 1996;66:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 124. | Yoshida K, Bolodeoku J, Sugino T, Goodison S, Matsumura Y, Warren BF, Toge T, Tahara E, Tarin D. Abnormal retention of intron 9 in CD44 gene transcripts in human gastrointestinal tumors. Cancer Res. 1995;55:4273-4277. [PubMed] |
| 125. | Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996;271:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 711] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 126. | Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 127. | Nakayama H, Yasui W, Yokozaki H, Tahara E. Reduced expression of nm23 is associated with metastasis of human gastric carcinomas. Jpn J Cancer Res. 1993;84:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994;56:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Akama Y, Yasui W, Yokozaki H, Kuniyasu H, Kitahara K, Ishikawa T, Tahara E. Frequent amplification of the cyclin E gene in human gastric carcinomas. Jpn J Cancer Res. 1995;86:617-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 130. | Yasui W, Kudo Y, Semba S, Yokozaki H, Tahara E. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn J Cancer Res. 1997;88:625-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Yasui W, Naka K, Suzuki T, Fujimoto J, Hayashi K, Matsutani N, Yokozaki H, Tahara E. Expression of p27Kip1, cyclin E and E2F-1 in primary and metastatic tumors of gastric carcinoma. Oncol Rep. 1999;6:983-987. [PubMed] |
| 132. | Suzuki T, Yasui W, Yokozaki H, Naka K, Ishikawa T, Tahara E. Expression of the E2F family in human gastrointestinal carcinomas. Int J Cancer. 1999;81:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 133. | Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, Moslein G, Baker SM, Liskay RM, Burgart LJ. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996;56:4836-4840. [PubMed] |
| 134. | Keller G, Grimm V, Vogelsang H, Bischoff P, Mueller J, Siewert JR, Höfler H. Analysis for microsatellite instability and mutations of the DNA mismatch repair gene hMLH1 in familial gastric cancer. Int J Cancer. 1996;68:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 135. | Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090-1095. [PubMed] |
| 136. | Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159-164. [PubMed] |
| 137. | Hamamoto T, Yokozaki H, Semba S, Yasui W, Yunotani S, Miyazaki K, Tahara E. Altered microsatellites in incomplete-type intestinal metaplasia adjacent to primary gastric cancers. J Clin Pathol. 1997;50:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Semba S, Yokozaki H, Yasui W, Tahara E. Frequent microsatellite instability and loss of heterozygosity in the region including BRCA1 (17q21) in young patients with gastric cancer. Int J Oncol. 1998;12:1245-1251. [PubMed] |
| 139. | Yasui W, Tahara H, Tahara E, Fujimoto J, Nakayama J, Ishikawa F, Ide T, Tahara E. Expression of telomerase catalytic component, telomerase reverse transcriptase, in human gastric carcinomas. Jpn J Cancer Res. 1998;89:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 140. | Yasui W, Tahara E, Tahara H, Fujimoto J, Naka K, Nakayama J, Ishikawa F, Ide T, Tahara E. Immunohistochemical detection of human telomerase reverse transcriptase in normal mucosa and precancerous lesions of the stomach. Jpn J Cancer Res. 1999;90:589-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 141. | Yasui W, Hata J, Yokozaki H, Nakatani H, Ochiai A, Ito H, Tahara E. Interaction between epidermal growth factor and its receptor in progression of human gastric carcinoma. Int J Cancer. 1988;41:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Yoshida K, Tsujino T, Yasui W, Kameda T, Sano T, Nakayama H, Toge T, Tahara E. Induction of growth factor-receptor and metalloproteinase genes by epidermal growth factor and/or transforming growth factor-alpha in human gastric carcinoma cell line MKN-28. Jpn J Cancer Res. 1990;81:793-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | Kuniyasu H, Yoshida K, Yokozaki H, Yasui W, Ito H, Toge T, Ciardiello F, Persico MG, Saeki T, Salomon DS. Expression of cripto, a novel gene of the epidermal growth factor family, in human gastrointestinal carcinomas. Jpn J Cancer Res. 1991;82:969-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Akagi M, Kawaguchi M, Liu W, McCarty MF, Takeda A, Fan F, Stoeltzing O, Parikh AA, Jung YD, Bucana CD. Induction of neuropilin-1 and vascular endothelial growth factor by epidermal growth factor in human gastric cancer cells. Br J Cancer. 2003;88:796-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Ito R, Kitadai Y, Kyo E, Yokozaki H, Yasui W, Yamashita U, Nikai H, Tahara E. Interleukin 1 alpha acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993;53:4102-4106. [PubMed] |
| 146. | Kitadai Y, Haruma K, Mukaida N, Ohmoto Y, Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin Cancer Res. 2000;6:2735-2740. [PubMed] |
| 147. | Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93-100. [PubMed] |
| 148. | Yoshida K, Yokozaki H, Niimoto M, Ito H, Ito M, Tahara E. Expression of TGF-beta and procollagen type I and type III in human gastric carcinomas. Int J Cancer. 1989;44:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 149. | Yamamoto S, Yasui W, Kitadai Y, Yokozaki H, Haruma K, Kajiyama G, Tahara E. Expression of vascular endothelial growth factor in human gastric carcinomas. Pathol Int. 1998;48:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Tanimoto H, Yoshida K, Yokozaki H, Yasui W, Nakayama H, Ito H, Ohama K, Tahara E. Expression of basic fibroblast growth factor in human gastric carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |