Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2945
Revised: October 28, 2005
Accepted: January 14, 2006
Published online: May 14, 2006
AIM: To discuss the clinical value of CT three-dimensional (3-D) imaging in diagnosing gastrointestinal tract diseases.
METHODS: Three-D imaging findings of 52 patients were retrospectively analyzed. Three-D imaging methods included shaded surface display (SSD), volume rendering (VR), virtual endoscopy (VE) and multiplanar reformatting (MPR). The diagnosis results of CT 3-D were evaluated by comparison with those of endoscopy and/or surgical finding.
RESULTS: Fifty-two patients with gastrointestinal tract diseases were diagnosed by CT 3-D imaging, of whom 50 cases were correctly diagnosed and 2 were misdiagnosed. There were 33 cases of gastric diseases (27 with carcinoma, 5 with peptic ulcer and 1 with leiomyoma) and 19 large intestinal diseases (10 with colon carcinoma, 2 with carcinoma of the rectum, 5 with colon polypus and 2 with tuberculosis of the ileocecal junction). Twenty-two cases with prominent lesions (9 with subsequent hollow lesions), 20 with stenosis of cavity (8 with concomitant prominent lesions) and 10 with hollow lesions (5 with concomitant prominent lesions) were shown in 3-D images. The minimal lesion shown was 1.0 cm × 0.8 cm × 0.5 cm.
CONCLUSION: CT 3-D imaging, a non-invasive examination without pain, can display clearly and directly the lesions of gastrointestinal tract with accurate location and high diagnosis accuracy. It is an important complementary technique to endoscopy.
- Citation: Duan SY, Zhang DT, Lin QC, Wu YH. Clinical value of CT three-dimensional imaging in diagnosing gastrointestinal tract diseases. World J Gastroenterol 2006; 12(18): 2945-2948
- URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2945
Barium studies and endoscopy are two conventional examinations of gastrointestinal tract diseases. In recent years, with the advancement of spiral CT (single-detector row spiral CT, SDCT) and multi-detector row spiral CT (MDCT) scanner, CT three-dimensional (3-D) imaging has gained wide application and high diagnosis accuracy in gastrointestinal tract diseases[1-4]. Here we report the results of CT 3-D imaging in diagnosing gastrointestinal tract diseases in our hospital, and evaluate its clinical application.
From Jan 2000 to Oct 2005, 52 patents with complete clinical data were examined by 3-D imaging (From Aug 2001 to Oct 2003, due to short supply of effervescent granules, no such examination was carried out), among whom 30 cases were males and 22 females. The average age of the patients was 57.3 years (range, 20-85 years). Thirty-three patients were examined in stomach and 19 in large intestine.
SDCT scanner (Somatom plus/4, Siemens, Germany) and workstation with 3-D imaging software (SUN magicview 1000) were used in 28 cases (18 in stomach and 10 in large intestine). MDCT scanner (LightSpeed/16, GE, America) and workstation with 3-D imaging software (Advantage Workstation 4.2) were used in 24 cases (15 in stomach and 9 in large intestine).
All the patients were required to fast for 12 h. The patient examined in the stomach took one pack (3 g) of effervescent granules (malu,Shanghai,China), lay in scanning bed and rolled to spread gas in stomach. A scout projection in supine position was made to confirm whether stomach was distended by gas. If distention was insufficient , another pack of effervescent granules (10 such cases in this study) or 500-1000 mL of 2%-3% Omnipaque 300 (Amersham, Shanghai,China) was added as an oral contrast agent to increase the contrasting effect and distending extent (8 such cases in this study). Another scout projection was scanned again. Holding breath for 10 s to 30 s, the patient was scanned with collimation of 5 mm (or 1.25 mm), pitch of 1.5 (or 1.375), increment of 2.4 mm (or 0.6 mm), scanning voltage of 120 kVp and a scanning current of 100-150 mA(the parameters in the brackets were used in scanning by MDCT). Another position of prone or lateral decubitus was often selected to better show some lesions (8 such cases in this study). The patients examined in the large intestines were asked to have a cleaning enema the night before and 30 min prior to the examination. A total of 600-1000 mL of air was infused through the anal canal as the contrasting agent. The following steps were the same as that used in stomach.
Three-dimensional imaging methods included surface display (SSD), volume rendering (VR), virtual endoscopy (VE) and multiplanar reformatting (MPR). By selecting reconstruction thresholds of gastrointestinal walls from 15 Hu to 40 Hu and gastrointestinal cavities from -200 Hu to -1500 Hu, and necessary cutting technique, we obtained satisfactory 3-D images on the workstation. The exhibition of gastrointestinal tract lesions on the 3-D images was analyzed and measured, and the diagnosis results of CT 3-D imaging were evaluated by comparison with those of endoscopy and/or surgical finding.
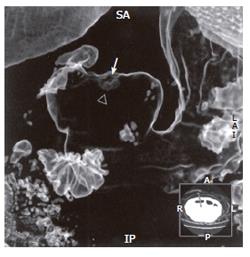
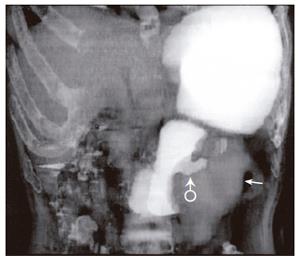
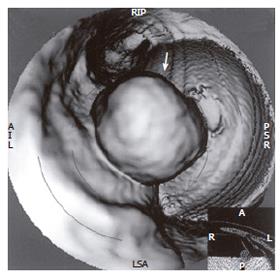
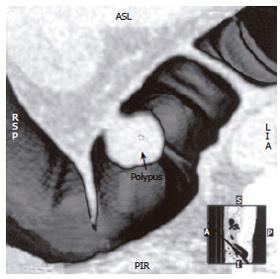
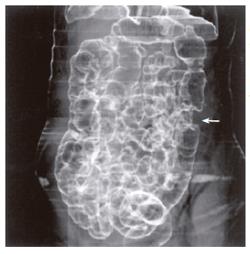
Fifty-two patients with gastrointestinal tract diseases were histologically proven, of whom 33 cases had gastric diseases (27 with carcinoma, 5 with peptic ulcer and 1 with leiomyoma) and 19 had intestinal diseases (10 with colon carcinoma, 2 with carcinoma of rectum, 5 with colon polypus and 2 with tuberculosis of ileocecal junction). Fifty cases were correctly diagnosed (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6) and 2 misdiagnosed (1 with leiomyoma in stomach and 1 with tuberculosis in ascending colon).
Prominent lesions (Figures 2-5) were shown in 3-D images in 22 cases (9 with subsequent hollow lesions, with maximal size of 5.4 cm × 6.0 cm × 2.1 cm and minimal of 1.5 cm × 1.2 cm × 0.8 cm), stenosis lesions (Figure 6) in 20 (8 with concomitant prominent lesions) and hollow lesions (Figure 1) in 10 (5 with concomitant prominent lesions, maximal of 2.0 cm × 1.8 cm × 1.1 cm, minimal of 1.0 cm × 0.8 cm × 0.5 cm). The locations, sizes and shapes of lesions in the 3-D images were close to that seen under endoscopy and/or in surgery.
Three-D images with VR, VE, SSD and MPR could show gastrointestinal tract lesions (Table 1), each with its own characteristics. SSD-imaging should be made with necessary cutting technique (Figure 5). VE-imaging could only show the extraluminal lesions. MPR-imaging had no perception of 3-D, but it could show clearly wall invasion (Figure 4). VR-imaging could show both extraluminal and intraluminal lesions (Figures 2, 6).
| Methods | Prominence (n=22) | Hollow (n=10) | Stenosis (n=20) | ||||||
| G | M | B | G | M | B | G | M | B | |
| SSD, cutting | 18 | 4 | 0 | 7 | 2 | 1 | 19 | 1 | 0 |
| V R | 20 | 2 | 0 | 9 | 1 | 0 | 19 | 1 | 0 |
| V E | 20 | 2 | 0 | 6 | 3 | 1 | 17 | 3 | 0 |
| MPR | 18 | 4 | 0 | 4 | 4 | 2 | 18 | 2 | 0 |
Gastrointestinal tract lesions could be clearly shown by 3-D imaging, and the exact location and accurate size of a lesion could be obtained when it was more than 5 mm. In our study, the minimal lesion shown by 3-D image was 1.0 cm × 0.8 cm × 0.5 cm. This result is accordant with that reported by Zhang and Gluecker[3,5]. The main advantages of 3-D imaging over two-dimensional (2-D) multiplanar reformatting are added perspective and enhanced depth perception. The relationship between the lesion and adjacent structures can be better appreciated. It is often helpful to use the combinations of different 3-D imaging methods in different lesions, and to start with 2-D multiplanar reformatting and then to proceed to 3-D imaging[6]. Therefore in order to increase the diagnostic accuracy, we suggest several 3-D images be observed as well as the original axial images.
Theoretically, the quality of 3-D images is related to slice thickening, pitch and tube current[7]. Our study found that a satisfactory 3-D image of increased spatial resolution and decreased moving artificial shadows was obtained with thinner collimations and appropriate tube current and pitch. Interference of the contents in the gastrointestinal tract was avoided and adequate distention was obtained by using gas from effervescent granules of air as the contrasting agent of gastrointestinal tract, or by setting the patient in the prone, lateral decubitus and left posterior oblique position[8]. If gastrointestinal tract is not well distended or gastrointestinal wall has collapsed, some lesions may be overlooked and some similar disease may appear[9]. Traditionally, low or high attenuation contrasting agent can both be taken to enhance and distend the gastrointestinal tract. As to whether to take low or high attenuation contrasting agent, we think low-attenuation, e.g. gas, should be the first choice, as the agents are safer, well tolerated, and always result in good gastrointestinal tract distention. 2%-3% Omnipaque 300 may be optimal when stomach cannot be well distended. Horton et al[1,10] reported low-attenuation agents, e.g. oil-based oral contrast agents, whole milk and water, resulted in satisfactory evaluation of detection of subtle diseases of the gastric wall, and did not interfere with the manipulation of 3-D data sets. It is necessary to conduct further study on the clinical application of oral contrast agents.
All the 3-D imaging methods could show the lesions of gastrointestinal tracts, each with its own characteristics: MPR image could show the lesion in any section, e.g. sagittal or oblique. Though it could not get 3-D perception, it could remedy the defects of transverse-images, with the advantages of showing the gastrointestinal lesions of wall invasion. Images of VE and SSD with cutting techniques can show the size and shape of the lesions[6], especially intraluminal prominent lesions, and show the structure of gastrointestinal tracts, e.g. semilunar fold and ileocecal valve, from any directions without being influenced by a narrow cavity. They can better show the base and pedicel of lesions, but cannot discern surface coloration of lesion and extraluminal tumor invading [11]. VR images can be made translucent, similar to those of double contrast barium studies, and can demonstrate the overall contour changes of gastrointestinal tracts, but with poor mucosal information[12].
Accurate staging of gastrointestinal cancer is essential because surgical resection is the treatment only for localized disease. Endoscopy and conventional barium study can only detect intraluminal lesions, but cannot evaluate extraluminal lesions. Traditional CT images can show anatomic structure and lesions of the gastrointestinal tract, but lack continuity and three dimensions. CT 3-D imaging has many advantages in the staging of primary tumor, assessing for local spread and detecting nodal involvement and distant metastases. It can provide valuable additional information and improve the detection and staging of both early and advanced gastric tumors and the detection of colorectal cancer and polypus[1,13,14]. CT 3-D imaging, with the same volume data obtained by spiral CT, especially by MDCT, can be repeatedly made with different reconstruction methods and avoid leakage of the section and moving artificial shadows. It has a high accuracy, sensitivity and specificity in showing prominent lesions and stenosis lesions, and in diagnosing gastric carcinoma and colon carcinoma[15]. 3-D imaging is a non-invasive examination without pain, and can easily be accepted by patients[14]. It may be used as an important complementary technique to endoscopy, particularly when the cavity is too narrow for endoscopy to go through. However, 3-D imaging examination has its limitations, e.g. it cannot show the color of lesions or make bioscopy[2].
CT 3-D imaging can clearly and directly show lesions of gastrointestinal tract. Diagnostic accuracy can be improved by observing different 3-D images together with original 2-D images. Three-D imaging is a non-invasive examination without pain, with characteristics of accurate localization of lesions and comprehensive observation of shapes. It is an important complementary technique to endoscopy.
S- Editor Wang J L- Editor Zhu LH E- Editor Bai SH
| 1. | Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003;23:625-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Zhang XP, Xu G, Xu Z, Sun YS. Evaluation of clinical application of three-dimensional CT in gastrointestinal tract. Zhonghua Fangshexue Zazhi. 2000;34:308-312. |
| 4. | Patak MA, Mortele KJ, Ros PR. Multidetector row CT of the small bowel. Radiol Clin North Am. 2005;43:1063-1077, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Gluecker TM, Fletcher JG, Welch TJ, MacCarty RL, Harmsen WS, Harrington JR, Ilstrup D, Wilson LA, Corcoran KE, Johnson CD. Characterization of lesions missed on interpretation of CT colonography using a 2D search method. AJR Am J Roentgenol. 2004;182:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Zhu Q, Shen L, Li J, Shan J, Song ZH, Li J, Yang BQ, Jin ML. [Three-dimensional spiral CT in advanced gastric cancer]. Zhonghua Zhongliu Zazhi. 2004;26:234-238. [PubMed] |
| 7. | McFarland EG, Brink JA, Loh J, Wang G, Argiro V, Balfe DM, Heiken JP, Vannier MW. Visualization of colorectal polyps with spiral CT colography: evaluation of processing parameters with perspective volume rendering. Radiology. 1997;205:701-707. [PubMed] |
| 8. | Kim SH, Lee JM, Han JK, Lee JY, Yang HK, Lee HJ, Shin KS, Choi BI. Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol. 2005;185:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Park SH, Ha HK, Kim MJ, Kim KW, Kim AY, Yang DH, Lee MG, Kim PN, Shin YM, Yang SK. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Horton KM, Fishman EK. Helical CT of the stomach: evaluation with water as an oral contrast agent. AJR Am J Roentgenol. 1998;171:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Chen F, Ni YC, Zheng KE, Ju SH, Sun J, Ou XL, Xu MH, Zhang H, Marchal G. Spiral CT in gastric carcinoma: comparison with barium study, fiberoptic gastroscopy and histopathology. World J Gastroenterol. 2003;9:1404-1408. [PubMed] |
| 12. | Ogata I, Komohara Y, Yamashita Y, Mitsuzaki K, Takahashi M, Ogawa M. CT evaluation of gastric lesions with three-dimensional display and interactive virtual endoscopy: comparison with conventional barium study and endoscopy. AJR Am J Roentgenol. 1999;172:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Röttgen R, Fischbach F, Plotkin M, Herzog H, Freund T, Schröder RJ, Felix R. Colon dissection: a new three-dimensional reconstruction tool for computed tomography colonography. Acta Radiol. 2005;46:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | You YT, Chang Chien CR, Wang JY, Ng KK, Chen JS, Tang R, Chiang JM, Yeh CY, Hsieh PS. Evaluation of contrast-enhanced computed tomographic colonography in detection of local recurrent colorectal cancer. World J Gastroenterol. 2006;12:123-126. [PubMed] |
| 15. | Inamoto K, Kouzai K, Ueeda T, Marukawa T. CT virtual endoscopy of the stomach: comparison study with gastric fiberscopy. Abdom Imaging. 2005;30:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |