Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2936
Revised: September 28, 2005
Accepted: October 26, 2005
Published online: May 14, 2006
AIM: To observe the effect of ischemic preconditioning on cyclinD1 expression in rat liver cells during early ischemic reperfusion.
METHODS: Fifty-four SD rats were randomly divided into ischemic preconditioning group (IP), ischemia/reperfusion group (IR) and sham operation group (SO). The IP and IR groups were further divided into four sub-groups (n = 6). Sham operation group (SO) served as the control group (n = 6). A model of partial liver ischemia/reperfusion was used, in which rats were subjected to liver ischemia for 60 min prior to reperfusion. The animals in the IP group underwent ischemic preconditioning twice for 5 min each time prior to the ischemia/reperfusion challenge. After 0, 1, 2, and 4 h of reperfusion, serum and liver tissue in each group were collected to detect the level of serum ALT, liver histopathology and expression of cyclinD1 mRNA and protein. Flow cytometry was used to detect cell cycle as the quantity indicator of cell regeneration.
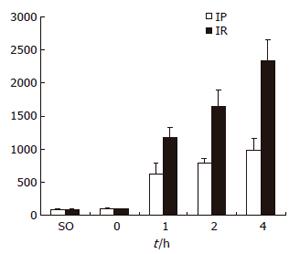
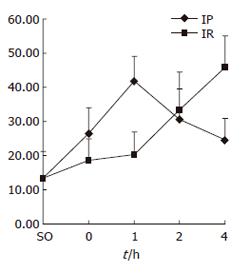
RESULTS: Compared with IR group, IP group showed a significantly lower ALT level in 1 h to 4 h sub-groups (P < 0.05). Proliferation index(PI) indicated by the S-phase and G2/M-phase ratio [(S+G2/M)/(G0/G1+S+G2/M)] was significantly increased in IP group at 0 and 1 h (26.44± 7.60% vs 18.56 ± 6.40%,41.87 ± 7.27% vs 20.25 ± 6.70%, P < 0.05). Meanwhile, cyclinD1 protein expression could be detected in IP group. But in IR group, cyclinD1 protein expression occurred 2 h after reperfusion. The expression of cyclinD1 mRNA increased significantly in IP group at 0 and 1 h (0.568 ± 0.112 vs 0.274 ± 0.069, 0.762 ± 0.164 vs 0.348 ± 0.093, P < 0.05).
CONCLUSION: Ischemic preconditioning can protect liver cells against ischemia/reperfusion injury, which may be related to cell proliferation and expression of cyclinD1 during early ischemic reperfusion.
- Citation: Cai FG, Xiao JS, Ye QF. Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats. World J Gastroenterol 2006; 12(18): 2936-2940
- URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2936
Hepatic ischemia/reperfusion (IR) injury is an important clinical problem occurring in hepatic resection or liver transplantation. Ischemic preconditioning (IP) has been proven to be an effective method protecting the liver against ischemia/reperfusion injury[1-3]. Many mechanisms are associated with this phenomenon[4-8], but some are still unclear. Previous studies showed that the analogous conditioning phenomenon induced by carbon tetrachloride (CCl4) is related to hepatocellular proliferation[9]. Whether IP promotes hepatocellular regeneration remains unknown. Changes in cell proliferation can be determined by cell cycle analysis. S-phase and G2/M-phase ratio is an indication the proliferation index (PI). Furthermore, cyclin and cyclin-dependent kinases (CDKs) are related to cell cycle regulation[10]. CyclinD1 is an important cyclin, which controls liver cell proliferation[11]. This study was to assess the effects of IP on cell regeneration and expression of cyclinD1 and to explore the possible mechanisms of IP protection.
Fifty-four male SD rats weighing 200-250 g, were randomly divided into ischemia group (IR group), ischemia preconditioning group (IP group) and sham operation group (SO). In IP group, each rat was subjected to 5 min ischemia, followed by 5 min reperfusion, twice, prior to 1 h hepatic ischemia. Specimens were taken after 0, 1, 2, and 4 h of reperfusion, respectively (n = 6). Before the experiment, all rats were fasted for 12 h and housed in a 12 h dark/12 h light cycle.
A 70% partial liver ischemia/reperfusion model was established according to the method reported by Ohmori et al[12]. After anesthesia with ketamine (40 mg/kg), the belly of each rat was cut open from the center. The portal vein and hepatic artery supplying the left and median lateral hepatic lobes were dissected and clamped with a small artery clamp. After 1 h ischemia, blood flow was restored by unclamping the vessels. After 0, 1, 2, and 4 h of reperfusion, serum and liver tissue of each group were collected to assess the level of serum ALT, liver histopathology, expression of cyclinD1 mRNA and protein. Liver tissue samples were homogenized and washed with PBS. The solution was filtered and centrifuged at 1000 r/min for 5 min. The cell pellet was resuspended in ice-cold ethanol to a final concentration of 80%. Flow cytometer was used to detect liver cell S-phase and G2/M-phase ratio as the quantity indicator of cell regeneration. In IP group, each rat was subjected to 5 min ischemia followed by 5 min reperfusion, twice, prior to 1 h hepatic ischemia. The other procedure was the same as in IR group. In SO group, each rat was only cut open from the center without any other procedure.
Liver tissue specimens were procured from the left lateral hepatic lobe, fixed with 10% neutral formaldehyde solution and embedded with paraffin. Sections were made and stained with H&E and observed under microscope.
Three mL of blood was withdrawn from the vena cava at certain time points, coagulated at room temperature, then centrifuged at 3000 r/min for 5 min for detecting the levels of alanine transaminase (ALT) with an automatic biochemistry analyzer (AU800, Olympus, Japan).
Single cell suspensions of hepatocytes were washed three times in PBS. RNase (1% w/v) was then added for 30 min at 37 °C and 50 mg/L PI (Sigma Company, Danmark) for 20 min. Samples were detected with a FACSORT flow cytometer (Becton Dickinson Company, USA), 10 000 cells were obtained by Cell Quest software and cell cycle was analyzed by Modfit software.
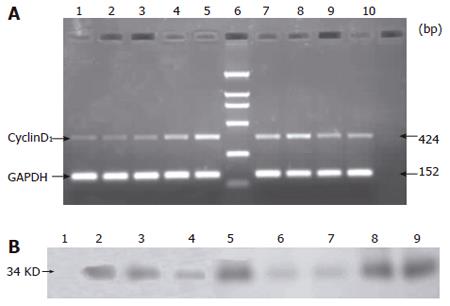
Primers synthesized according to the GenBank of Bioya Biotechnology Limited Company(Shanghai, China) included cyclinD1 sense primer (5’-TGGAGCCCCTGA AGAAGAG) cyclinD1 anti-sense primer (5’- AAGTGCGTTGTGCGGTAGC -3’) amplified products (424bp), GAPDH sense primer (5’-GGCTGAGAATGGGAAGCTG-GTCAT-3’) and GAPDH anti-sense primer (5’- CAGCCTTCTCCATGGTGGTGAA- GA-3) amplified products (152bp).
Total RNA was extracted from the frozen liver specimens with Trizol (GibcoBrl Company, USA). Reverse transcription synthesis of cDNA was carried out according to the instructions of SuperScript One-Step RT-PCR with Platinum Taq kit (Invitrogen Company, USA). PCR was performed with a Gene Amp PCR System 2400 (PE Company, USA). The reaction system was composed of a cDNA template (1.0 μL), 2×buffer (12.5 μL), 5 mmol/L MgCl2(2.5 μL), cyclin D1, GAPDH sense and anti-sense primer (0.5 μL respectively), SSRT polymerase (0.5 μL) and deionized water (6.5 μL). The reaction system was put into a PCR machine for amplification. Cyclin D1 and GAPDH were amplified for 35 cycles of PCR. The amplification conditions were at 55 °C for 30 min, at 94 °C for 2 min, at 94 °C for 30s, at 55 °C for 30 s, at 72 °C for 45 s, a final extension at 72 °C for 7min. The amplified products were preserved at 4 °C. Ten μL of PCR products was electrophoresed on 1.5% agarose gel containing 5 μg/mL ethidium bromide (Sigma Company, USA). Marker was DL-2000 DNA marker. The results were detected by GeneSnap (SynGene, England) and analyzed by GeneTools (SynGene, England).
Liver tissues were homogenized in 10 volumes of ice-cold buffer containing 50 mmol/L Tris-HCl (pH7.5), 150mmol/L NaCl, 1%Triton-X 100, 1 mmol/L EDTA,1mmol/L DTT,10μg/mL pepstatin,10μg/mL leupeptin, 10 μg/mL aprotinin, 1mM PMSF. The homogenates were centrifuged at 12 000 r/min for 15 min at 4 °C. The supernatant was frozen and stored at -80 °C for further analysis. After measurement of the supernatant sample concentration, the sample was mixed with an equal volume buffer containing 0.1mol/LTris (pH 6.8), 100 mmol/L DTT, 2% SDS, 10% glycerin, 0.01% bromophenol blue and put in water bath to boil for 3-5 min. Fifty micrograms of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The stacking and resolving gels were composed of 4% and 10% polyacrylamide, respectively. After SDS-PAGE, the gels were electroblotted onto nitro-cellulose (NC) membranes at 100 mA for 2 h. The membranes were blocked for 1 h in blocking solution at room temperature, which contained 1 mL/L Tween 20 (PBS-T) and 50 g/L non-fat dry milk. Then they were immunolabeled with the primary antibody (1:500 rabbit anti-rat Santa Cruz Company, USA) in blocking solution for 1 h at room temperature and was washed three times with TBST for 15 min. The NC membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:1000 goat anti-rabbit Santa Cruz Company, USA) for 1 h at room temperature and washed three times with TBST for 15 min again. Color solution of diaminobenzidine (DAB) was prepared according to the manufacturer’s directions (Maxim Company, China). After incubation with this color solution, the protein bands were visible on the NC membranes.
The results were presented as mean ± SD. Multivariate analysis of variance and t test were performed by SPSS11.5 for window. P < 0.05 was considered statistically significant.
Swelling of hepatocytes and structural derangement were observed in IR group during the early period of reperfusion. Spot necrosis, hemorrhage and inflammatory cell infiltration could be seen in hepatic lobes or around central veins, with extended reperfusion time. Compared with IR group, IP group showed moderate hepatocyte swelling and less necrotic area.
The levels of serum ALT increased gradually as reperfusion time was extended in both groups. Compared with IR group, the level of serum ALT in IP group was significantly lower at all time points after reperfusion (P < 0.05) (Figure 1).
PI increased significantly at 0 and 1 h of reperfusion in IP group compared with IR group. There was a statistically significant difference (P < 0.05) (Figure 2).
The RT-PCR results showed that cyclinD1 mRNA was expressed in SO, IR and IP groups. The expression of cyclinD1 mRNA in IP group increased at 0-1 h after reperfusion. Compared with IP group, the expression of cyclinD1 mRNA in IR group was significantly lower at 0-1 h after reperfusion (P < 0.05). No significant difference in the expression of cyclinD1 mRNA was found between SO and IR groups at 0-1 h after reperfusion (P > 0.05, Table 1, Figure 3A).
CyclinD1 protein was not detectable until 2 h after reperfusion in IR and SO groups. Whereas cyclinD1 protein was expressed in IP group soon after the reperfusion was begun (Figure 3B).
It is widely accepted that IP protects organs against IR injury. It has been reported that IR injury is related with many mechanisms, such as suppression of inflammatory reaction[13], improvement of local microcirculation[14], diminution of cell apoptosis and decreased production of local active oxygen-derived free radicals[15,16]. But some of the mechanisms remain uncertain. To probe into the mechanisms of IP protection may help to find an effective method against IR injury. In this study, we adopted the rat model of ischemic preconditioning against subsequent hepatic IR injury and confirmed that it could reduce the levels of serum ALT and the extent of liver necrosis.
Application of flow cytometry to cell cycle analysis makes it possible to analyze cell proliferation. Cell cycle can discriminate cells at the G0/G1-phase, S-phase and G2/M-phase. It is considered that cells at the S-phase and G2/M-phase can proliferate. S-phase and G2/M-phase ratio could indicate the proliferation index (PI).The finding of the present work is that IP could promote liver cell proliferation during early ischemic reperfusion, which is possibly related to IP protection. Zhang et al[17] showed that proliferating liver cells can resist IR injury. The mechanisms of cellular proliferation involved in IP are still obscure, but may be related to reactive oxygen species (ROS) and tumor necrosis facter-α (TNF-α).The liver underlying IP could generate ROS and TNF-α during early reperfusion[18,19]. ROS could stimulate cell mitosis and proliferation[20]. TNF-alpha is a known mitogen for hepatocytes and an important factor for liver cell proliferation. In the presence of anti-TGF-alpha neutralizing Abs, the mitogenic activity of TNF-alpha is abrogated[21].
Breakthrough in cell cycle regulation is the understanding of cyclin and cyclin-dependent kinase, which regulates cell cycle progression. Mitotic cellular division requires cells to leave the resting state (G0/G1) and proceed through the phase of DNA synthesis (S) and mitosis (G2/M). There is a check point between G0/G1-phase and S-phase. CyclinD1 is an important cyclin which can make cells pass through the check point and enter S-phase and G2/M-phase. Higher expression of cyclinD1 could promote cell proliferation in rat liver[11]. Our data showed that IP could stimulate cyclinD1 mRNA and protein expression during the early reperfusion in IP group and not in IR group. The mechanisms may be related to the fact that IP activates the p38MAPK pathway[22], leading to activation of regulatory proteins and production of DNA synthetic proteins, such as cyclinD1 and interleukin-6(IL-6)[23]. The p38MAPK pathway is an important signaling pathway involved in IP. Blockade of the p38MAPK pathway can lead to loss of protection of IP[24]. When the p38MAPK pathway is activated, the p38kinase transfers to cell nuclei and induces the production of cyclinD1[25]. In our study, the cyclinD1 protein was expressed in IR group 2 h after reperfusion, although cyclinD1 mRNA was expressed during the early reperfusion. In SO group, cyclinD1 mRNA was expressed but cyclinD1 protein was not expressed. These data suggest that cyclinD1 expression is posttranscriptionally regulated. Awad and Gruppuso[26] showed that a posttranscriptional mechanism regulating cyclin D1 content is involved in temporary hepatocyte growth arrest. They speculated that this posttranscriptional regulation may be downstream from the p38 mitogen-activated protein kinase pathway. The posttranscriptional regulation could keep adult hepatocytes in a quiescent state, but the mechanism is not completely clear.
In conclusion, IP could promote cyclinD1 expression and lead to hepatocellular proliferation during early ischemic reperfusion, which is possibly one of the protective mechanisms against IR injury. However their underlying regulatory pathway and biological role remain unclear.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Lloris-Carsí JM, Cejalvo D, Toledo-Pereyra LH, Calvo MA, Suzuki S. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc. 1993;25:3303-3304. [PubMed] |
| 2. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Sawaya DE Jr, Brown M, Minardi A, Bilton B, Burney D, Granger DN, McDonald JC, Zibari GB. The role of ischemic preconditioning in the recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion. J Surg Res. 1999;85:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163-G171. [PubMed] |
| 7. | Peralta C, Perales JC, Bartrons R, Mitchell C, Gilgenkrantz H, Xaus C, Prats N, Fernández L, Gelpí E, Panés J. The combination of ischemic preconditioning and liver Bcl-2 overexpression is a suitable strategy to prevent liver and lung damage after hepatic ischemia-reperfusion. Am J Pathol. 2002;160:2111-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Cai ZW, Mehendale HM. Protection from CCl4 toxicity by prestimulation of hepatocellular regeneration in partially hepatectomized gerbils. Biochem Pharmacol. 1991;42:633-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1527] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 11. | Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397-404. [PubMed] |
| 12. | Ohmori M, Miyashita F, Uchida H, Kitoh Y, Tsuruoka S, Harada K, Sugimoto K, Fujimura A, Kobayashi E. Effect of erythromycin on ischemia-reperfusion injury of liver in rats. Transplant Proc. 2000;32:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Choukèr A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, Peter K, Thiel M. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg. 2005;140:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Vajdová K, Heinrich S, Tian Y, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping improve murine hepatic microcirculation and Kupffer cell function after ischemic injury. Liver Transpl. 2004;10:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825-1828. [PubMed] |
| 17. | Zhang BH, Gong DZ, Mei MH. Protection of regenerating liver after partial hepatectomy from carbon tetrachloride hepatotoxicity in rats: role of hepatic stimulator substance. J Gastroenterol Hepatol. 1999;14:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Rüdiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1705] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 21. | Gallucci RM, Simeonova PP, Toriumi W, Luster MI. TNF-alpha regulates transforming growth factor-alpha expression in regenerating murine liver and isolated hepatocytes. J Immunol. 2000;164:872-878. [PubMed] |
| 22. | Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fraunberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1244] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 26. | Awad MM, Gruppuso PA. Cell cycle control during liver development in the rat: evidence indicating a role for cyclin D1 posttranscriptional regulation. Cell Growth Differ. 2000;11:325-334. [PubMed] |