Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2914
Revised: November 28, 2005
Accepted: February 10, 2006
Published online: May 14, 2006
AIM: Microcirculatory dysfunction and free oxygen radicals are important factors in the pathogenesis of severe acute pancreatitis. Additional oxygen delivery might enhance lipid peroxidation but may also improve pancreatic microcirculation. This study assesses the effect of free cellular bovine hemoglobin on the formation of oxygen radicals and microcirculation in a rodent model of severe acute pancreatitis.
METHODS: Fifteen minutes after induction of acute pancreatitis Wistar rats received either 0.8 mL bovine hemoglobin (HBOC-200), hydroxyethyl starch (HES) or 2.4 mL of normal saline to ensure normovolemic substitution. After 6 h of examination the pancreas was excised and rapidly processed for indirect measurement of lipid peroxidation products malondialdehyde (MDA) and reduced glutathione (GSH) in pancreatic tissue.
RESULTS: The single application of HBOC-200 improved pancreatic microcirculation and reduced histopathological tissue damage significantly. Tissue concentration of MDA did not differ between the groups. Also no differences in GSH levels were detected.
CONCLUSION: Though the single application of HBOC-200 and HES improve pancreatic microcirculation, no differences in lipid peroxidation products were detected. The beneficial effect of additional oxygen supply (HBOC-200) does not lead to enhanced lipid peroxidation.
- Citation: Kleinhans H, Mann O, Schurr PG, Kaifi JT, Hansen B, Izbicki JR, Strate T. Oxygen radical formation does not have an impact in the treatment of severe acute experimental pancreatitis using free cellular hemoglobin. World J Gastroenterol 2006; 12(18): 2914-2918
- URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2914.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2914
Microcirculatory dysfunction and free oxygen radicals are important factors in the pathogenesis of acute experimental pancreatitis[1-3]. Oxygen free radicals are formed during aerobic cellular metabolism. Under physiological conditions they are eliminated by a system of enzymatic and non-enzymatic antioxidants. Under conditions of imbalance like in acute pancreatitis between antioxidants and oxidants as in hyperoxygenation, ischemia and reperfusion and tissue inflammation “oxidative stress” occurs[4-8].
Oxygen species initiate a pronounced peroxidation of membrane lipids, detectable after 30 min by a significant rise in the lipid peroxidation marker malondialdehyde (MDA), persisting up to 16 h[8,9]. At the same time a significant decrease in glutathione thiols (GSH, potent antioxidant in the pancreas) occurs[10,11]. In the human, acute pancreatitis increases the amount of lipid peroxidation products and decreases antioxidants[12-14].
It has been shown that pancreatic microcirculatory deficits can be treated with bovine haemoglobin, which improves fluidity due to its colloid effects and delivers cell free oxygen to oxygen deprived tissue[15]. However, additional application of cell free oxygen might potentially enhance detrimental O2 radical products[16], which could limit the positive value of bovine haemoglobin in the treatment of severe acute pancreatitis.
This study assesses the relationship between free oxygen radicals and pancreatic microcirculation in a rodent model of severe acute pancreatitis after therapeutic application of a hemoglobin based oxygen carrier (HBOC).
The experimental protocol was approved by the Ethical Committee of the Hamburg Federal Board of Veterinary Medicine and Animal Care. After overnight fasting with free access to water containing 20% glucose, thirty female Wistar rats (230-250 g) were randomly assigned to three groups (n = 10). Anesthesia was induced by intraperitoneal pentobarbital (40 mg/kg BW) and ketamine (10 mg/kg BW) allowing spontaneous breathing by tracheostomy. Ringer lactate was infused for fluid resuscitation keeping the central venous pressure between 4 and 6 mmHg and heart rate as well as mean arterial pressure within 10% of baseline frequency throughout the experiment (Datex AS/3 monitoring system; Hoyer, Bremen, Germany).
Acute pancreatitis was induced according to a standardised regimen[17] using a combination of intravenous cerulein infusion (5 mg/kg per hour; Takus, Pharmacia, Erlangen, Germany) at a rate of 1 mL/h over 6 h and intraductal infusion of glycodeoxycholic acid (GDOC: 10 mmol/L; 1 mL/kg for 300 s; infusion pressure 25 to 30 mmHg).
Fifteen minutes after induction the animals received either 0.8 mL bovine hemoglobin (HBOC-group) (HBOC-200, Biopure MD, USA), 0.8 mL hydroxyethyl starch (HES-group 3) or 2.4 mL of normal saline (NaCl-group) to ensure normovolemic substitution. In vivo fluorescence microscopy was performed with an epi-illumination unit (Zeiss, Germany). Microcirculation was observed for 6 h, measurements were performed in the head of the pancreas: 0 min was defined as the intraductal infusion of GDOC. Leucocyte adherence (area of adherent leucocytes in a percentage of the vein cross section) and functional capillary density (number of perfused capillaries to the total number of capillaries) were studied. After 6 h of examination the animals were sacrificed by an intravenous injection of pentobarbital. Pancreatic tissue samples were excised for determination of tissue damage and malondialdehyde (Lipid Peroxidation Assay Kit, Calbiochem, San Diego, USA) and GSH concentrations (Glutathion Assay Kit, Calbiochem, San Diego, USA) specified in μmol/L/g protein. The samples were immediately processed and stored at -80 °C. The Glutathione Assay Kit takes advantage of a 2-step reaction. The first step involves the formation of thioethers when a patented reagent reacts with all mercaptans (including glutathione) in the sample. The second step is an elimination reaction that takes place in alkaline conditions. This second reaction is mediated by 30% NaOH which specifically transforms the substitution product obtained with GSH into a chromophoric thione with a maximal absorbance at 400 nm. The main advantage of the method is that it does not require any enzyme as a reagent.
The Lipid Peroxidation Assay Kit takes advantage of a chromogenic reagent (10.3 mmol/L N-methyl-2-phenylindole, in acetonitrile) which reacts with MDA and 4-hydroxyalkenals at 45 °C. Condensation of one molecule of either MDA or 4-hydroxyalkenal with 2 molecules of 10.3 mmol/L N-methyl-2-phenylindole yields a stable chromophore with maximal absorbance at 586 nm.
Tissue protein concentration was detected by using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). The BCA Protein Assay is a detergent-compatible formulation based on bicinchoninic acid (BCA) for the colometric detection and quantitation of total protein. This method combines the reduction of Cu+2 to Cu+1 by protein in an alkaline medium (the biuret reaction) with the colometric detection of the cuprous cation (Cu+1) using a unique reagent containing bicinchonic acid[18].
The histopathological scoring was analyzed by light microscopy using a validated score quantifying acinar necrosis, fat necrosis and hemorrhage, leucocyte infiltration and edema[19].
Statistical analysis of the changes from baseline values in each group of animals utilized the paired Student's t-test. Significances between the groups were assessed by the unpaired t-test. P < 0.05 was considered significant. All data are presented as mean ± SD.
Results of pancreatic microcirculation and tissue damage have been reported elsewhere[15].
In essence pancreatic microcirculation improved in the HBOC-200-group when compared to NaCl-group [functional capillary density (fcd): 0.7 (SD 0.3) vs 0.33 (0.12); P < 0.05]; leucocyte adherence (la): 0.27 (0.14) vs 0.54 (0.19); P < 0.05). HES also improved pancreatic microcirculation when compared to NaCl-group (fcd: 0.49 (0.15) vs 0.33 (0.12); P < 0.05 and la: 0.39 (0.11) vs 0.54 (0.19); P < 0.05).
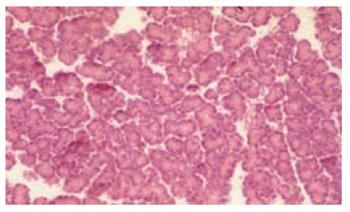
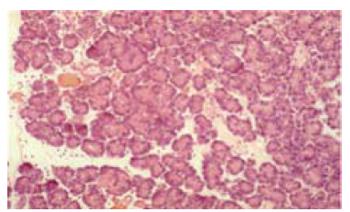
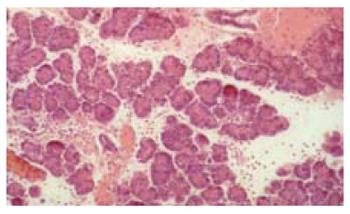
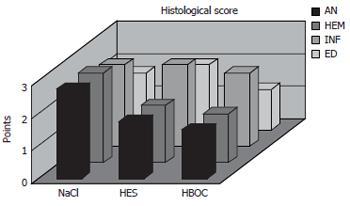
The histological score showed significantly less tissue damage in HBOC-group (Figure 1) versus NaCl-group (6.25 vs 9.25) (range 3-8.5 vs 8-10.75); P < 0.001. The overall score is reflected within all subgroups. Comparing HES and HBOC-therapy significantly less tissue damage was found (6.25 vs 8) (range 3-8.5 vs 6.5-10.25); P = 0.006 in the HBOC group. When comparing HES (Figure 2) and NaCl (Figure 3) less necrosis and hemorrhaging (1.75 vs 2.75) (range 1.5-2.75 vs 2.25-3.5) was found in the HES therapy group(Figure 4).
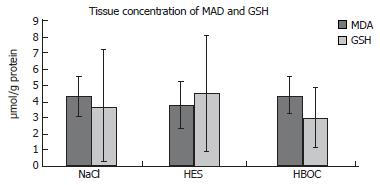
Tissue concentration of MDA did not differ between the groups (HBOC-200-group: 4.37 ± 1.11 μmol/L per g protein; HES-group: 3.77 ± 1.45 μmol/L per g protein; NaCl-group: 4.31 ± 1.19 μmol/L per g protein. MDA-tissue levels (n = 10) of sham operated rats were 5.41 ± 2.12 μmol/L per g protein.
Also no differences in GSH levels were detected HBOC-group: 2.93 ± 1.72 μmol/L per g; HES-group: 4.46 ± 3.55 μmol/L per g; NaCl-group: 3.7 ± 3.5 μmol/L per g. Control GSH-tissue levels (n = 10) of sham operated rats were 12.19 ± 7.04 μmol/L per g protein (Figure 5).
Clinical investigations with free hemoglobin solutions have been performed since the late 1800 s. Over the years complications like renal toxic effects and short circulation time have been reduced. The new blood substitutes are able to replace blood, colloid and crystalloid solutions act as oxygen transporter in many experimental settings. The ability of potent oxygen transport to oxygen deprived tissue has been shown in different animal models[15,20].
Vincent et al investigated the influence of heme-binding proteins in heme-catalyzed oxidations. They confirmed previous studies that heme acts as a catalyst of H2O2-dependent lipid peroxidation[21]. It has been shown that diene conjugation caused by oxygen radicals gives an adequate assessment of lipid peroxidation in tissue extracts. Several studies emphasize the role of oxygen free radicals in the pathogenesis of experimental acute pancreatitis[1,3,22-26]. Oxygen radicals are generated by different biological systems. Sources are reperfusion injury as well as the respiratory burst of PMN-leucocytes[27-30]. Local tissue damage has successfully been treated by the use of radical scavengers[31,32]. However increased heme and oxygen availability like in HBOC-therapy might lead to higher generation of oxygen radicals and tissue damage caused by lipid peroxidation.
A single application of a hemoglobin based oxygen carrier in this model of severe acute pancreatitis reduces the tissue damage and ensures a stable microcirculation. Colloids like HES and Dextrane have also been proven to be an effective therapy for microcirculatory dysfunctions[33-36]. The superiority of HBOC[34,36,37] might be explained by the combining effect of a colloid and noncorpuscular oxygen carrier. HBOC releases oxygen in areas of poor perfundation and ensure tissue oxygenation.
However, the hypothesis that hyperoxygenation and additional oxygen supply might lead to an increased production of oxygen free radicals was not supported by this data. This study could not confirm former publications suggesting that increased heme availability promotes the enhencement of lipid peroxidation. Dunne et al demonstrated the reactivity of various modified hemoglobins to hydrogen peroxide in terms of free radical formation. They compared PHP hemoglobin (crosslinked between the β-subunits and conjugated with polyoxyethylene) with DBBF hemoglobin (crosslinked between the β-subunits using dibromosalicylfumerate, and control HbA0. All the blood substitutes generated free radicals. MDA- levels were equal in nearly all groups. When compared to sham operated animals MDA-levels in the HBOC groups reached the same levels. One explanation of this finding could be that the level of MDA and GSH might have reached their peak within the first hours of pancreatitis and decreased to normal levels after 6 h. This theory is underlined by former studies[23,32,38-39] Schoenberg et al determined the peroxidation products, conjugated dienes and MDA in hemorrhagic pancreatitis induced by retrograde injection of sodium-taurocholate . The tissue levels of MDA increased, reaching their highest level after 1 h. Two hours later the lipid peroxides had returned to control levels.
GSH-tissue levels also showed no statistically relevant differences in the groups. Compared to GSH-levels of sham operated rats a decrease was found which might be an indication for oxidative stress in all groups. Dabrowski et al were able to show a decrease in pancreatic GSH at an early stage of cerulein-induced pancreatitis in rats[40,41].
Other researchers were able to prevent local tissue damage in experimental pancreatitis by the use of radical scavenging therapy[26,31-32]. Since we could not detect differences in oxidative stress markers we would explain the reduced histopathological damage by an improved pancreatic microcirculation. An excess formation of free oxygen radicals in the setting of additional oxygen supply was not detected.
S- Editor Wang J L- Editor Zhang JZ E- Editor Bi L
| 1. | Dabrowski A, Gabryelewicz A. Oxidative stress. An early phenomenon characteristic of acute experimental pancreatitis. Int J Pancreatol. 1992;12:193-199. [PubMed] |
| 2. | Koiwai T, Oguchi H, Kawa S, Yanagisawa Y, Kobayashi T, Homma T. The role of oxygen free radicals in experimental acute pancreatitis in the rat. Int J Pancreatol. 1989;5:135-143. [PubMed] |
| 3. | Sanfey H, Sarr MG, Bulkley GB, Cameron JL. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109-118. [PubMed] |
| 4. | Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 307] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991;69:965-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291-295. [PubMed] |
| 7. | Halliwel B, Gutteridge JM. Free radicals, aging and diseases. ed 2. Oxford: Clarendon Press 1989; 416-508. |
| 8. | Weber H, Merkord J, Jonas L, Wagner A, Schröder H, Käding U, Werner A, Dummler W. Oxygen radical generation and acute pancreatitis: effects of dibutyltin dichloride/ethanol and ethanol on rat pancreas. Pancreas. 1995;11:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Weber H, Roesner JP, Nebe B, Rychly J, Werner A, Schröder H, Jonas L, Leitzmann P, Schneider KP, Dummler W. Increased cytosolic Ca2+ amplifies oxygen radical-induced alterations of the ultrastructure and the energy metabolism of isolated rat pancreatic acinar cells. Digestion. 1998;59:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Luethen R, Quante M, Schoepp C, Haussinger D, Niederau C. Auswirkungen der Arginin-Pankreatitis auf den Thiol- und NO. Metabolismus des Pankreas. Z Gastroenterol. 1996;34:624. |
| 11. | Luthen R, Quante M, Schoepp C, Haussinger D, Niederau C. Thiolmetabolismus und oxidativer Stress in der Fruehphase einer experimentellen Pankreatitis. Hepato-Gastroenterology. 1999;46:2751-2756. |
| 12. | Guyan PM, Uden S, Braganza JM. Heightened free radical activity in pancreatitis. Free Radic Biol Med. 1990;8:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Schoenberg MH, Büchler M, Pietrzyk C, Uhl W, Birk D, Eisele S, Marzinzig M, Beger HG. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995;10:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Schoenberg MH, Birk D, Beger HG. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62:1306S-1314S. [PubMed] |
| 15. | Strate T, Mann O, Kleinhans H, Schneider C, Knoefel WT, Yekebas E, Standl T, Bloechle C, Izbicki JR. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg. 2003;238:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Simoni J, Feola M, Canizaro PC. Generation of free oxygen radicals and the toxicity of hemoglobin solutions. Biomater Artif Cells Artif Organs. 1990;18:189-202. [PubMed] |
| 17. | Bloechle C, Kusterer K, Kuehn RM, Schneider C, Knoefel WT, Izbicki JR. Inhibition of bradykinin B2 receptor preserves microcirculation in experimental pancreatitis in rats. Am J Physiol. 1998;274:G42-G51. [PubMed] |
| 18. | Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16085] [Cited by in RCA: 16142] [Article Influence: 403.6] [Reference Citation Analysis (0)] |
| 19. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 672] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Standl T, Horn P, Wilhelm S, Greim C, Freitag M, Freitag U, Sputtek A, Jacobs E, Schulte am Esch J. Bovine haemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can J Anaesth. 1996;43:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988;265:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Closa D, Bulbena O, Hotter G, Roselló-Catafau J, Fernández-Cruz L, Gelpí E. Xanthine oxidase activation in cerulein- and taurocholate-induced acute pancreatitis in rats. Arch Int Physiol Biochim Biophys. 1994;102:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nonaka A, Manabe T, Tamura K, Asano N, Imanishi K, Tobe T. Changes of xanthine oxidase, lipid peroxide and superoxide dismutase in mouse acute pancreatitis. Digestion. 1989;43:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984;200:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Schoenberg MH, Büchler M, Helfen M, Beger HG. Role of oxygen radicals in experimental acute pancreatitis. Eur Surg Res. 1992;24 Suppl 1:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Steer ML, Rutledge PL, Powers RE, Saluja M, Saluja AK. The role of oxygen-derived free radicals in two models of experimental acute pancreatitis: effects of catalase, superoxide dismutase, dimethylsulfoxide, and allopurinol. Klin Wochenschr. 1991;69:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978;298:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1699] [Cited by in RCA: 1591] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 28. | Coticchia JM, Lessler MA, Ellison EC, Carey LC. Mitochondrial dysfunction induced by pancreatitis-associated ascitic fluid. Proc Soc Exp Biol Med. 1983;172:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Southorn PA, Powis G. Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc. 1988;63:390-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Southorn PA, Powis G. Free radicals in medicine. I. Chemical nature and biologic reactions. Mayo Clin Proc. 1988;63:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Poch B, Gansauge F, Rau B, Wittel U, Gansauge S, Nüssler AK, Schoenberg M, Beger HG. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999;461:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, Bültmann B, Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Horn EP, Standl T, Wilhelm S, Jacobs EE, Freitag U, Freitag M, Schulte am Esch J. [Bovine hemoglobin. HBOC-201 causes a reduction of the oxygen partial pressure in poststenotic skeletal muscle]. Anaesthesist. 1998;47:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Hotz HG, Schmidt J, Ryschich EW, Foitzik T, Buhr HJ, Warshaw AL, Herfarth C, Klar E. Isovolemic hemodilution with dextran prevents contrast medium induced impairment of pancreatic microcirculation in necrotizing pancreatitis of the rat. Am J Surg. 1995;169:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Klar E, Herfarth C, Messmer K. Therapeutic effect of isovolemic hemodilution with dextran 60 on the impairment of pancreatic microcirculation in acute biliary pancreatitis. Ann Surg. 1990;211:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Klar E, Foitzik T, Buhr H, Messmer K, Herfarth C. Isovolemic hemodilution with dextran 60 as treatment of pancreatic ischemia in acute pancreatitis. Clinical practicability of an experimental concept. Ann Surg. 1993;217:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Horn EP, Standl T, Wilhelm S, Jacobs EE, Freitag U, Freitag M, Schulte am Esch J. Bovine hemoglobin increases skeletal muscle oxygenation during 95% artificial arterial stenosis. Surgery. 1997;121:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Schoenberg MH, Büchler M, Schädlich H, Younes M, Bültmann B, Beger HG. Involvement of oxygen radicals and phospholipase A2 in acute pancreatitis of the rat. Klin Wochenschr. 1989;67:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Dabrowski A, Chwiećko M. Oxygen radicals mediate depletion of pancreatic sulfhydryl compounds in rats with cerulein-induced acute pancreatitis. Digestion. 1990;47:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Dabrowski A, Gabryelewicz A, Wereszczyńska-Siemiatkowska U, Chyczewski L. Oxygen-derived free radicals in cerulein-induced acute pancreatitis. Scand J Gastroenterol. 1988;23:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |