INTRODUCTION
Disorders of the gastrointestinal (GI) tract may be examined using endoscopy, X-ray, radionuclide studies, electromyography, magnetic resonance imaging (MRI) and manometry. Specific analysis of structural changes occurring within the GI wall is, however, difficult to study by these techniques. Intraluminal ultrasonography (endosonography (ES), endoscopic ultrasonography (EUS) is the common denomination of ultrasound examinations using intracorporal transducers which are inserted into the GI tract. Thus, the visceral wall and adjacent structures can be imaged in detail[1-3]. Acoustic coupling is usually achieved by filling water into the lumen or into a balloon around the ultrasound transducer. Alternatively, the ultrasound probe can be applied directly on the endoluminal surface.
Endosonography is currently mostly performed in the upper GI tract and rectum. However, endosonographic imaging of the colon and distal ileum as well as intraductal ultrasonography (IDUS) of biliary and pancreatic ducts are frequently performed with miniprobes. Dedicated echocolonoscopes are also available, but these have so far gained limited clinical application.
ENDOSONOGRAPHIC INSTRUMENTS
Echoendoscopes combine endoscopy with integrated ultrasound transducers. These instruments can be classified both according to the type of endoscope and the US system. Echoendoscopes may have radial or curvilinear array US transducers using frequencies between 7.5 and 30 MHz. These systems are being continuously improved. The possibility to combine echoendoscopes with conventional ultrasound machines has allowed the use of different ultrasound modalities like B-mode, M-mode and Doppler technology. Ultrasound elastography is a new method with the potential of separating normal from pathological tissue. Recently, elastography and contrast enhanched ultrasound have been applied also in endosonographic imaging[4]. US miniprobes have a diameter which allows the probe to be inserted through the biopsy channel of a conventional endoscope or a suitable catheter (Figure 1). Currently, available US miniprobes comprise mechanical sector, electronic phased arrays, or single crystal linear compound systems operating with frequencies between 12 and 30 MHz. Systems that combine linear compound scanning with mechanical sector scanning or M-mode registration are also available making it possible to switch between these modalities.
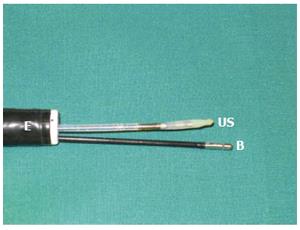
Figure 1 An endosco-pe with two channels.
A miniature ultrasound transducer (US) and a biopsy forceps (B) are inserted through the channels.
The small size and high frequencies of miniature probes reduce the size of the US image and the penetration depth of the US beam into the tissue as compared to echoendoscopes. However, small diameter probes can be passed into stenotic areas not traversable with conventional echoendoscopes. Doppler sonography may provide important information about vascular flow and separate vessels from other echo-poor structures. Some echoendoscopes have integrated Doppler technology and Doppler miniprobes for transendoscopic use have also been developed[5]. Other ultrasound systems designed for use within the GI tract do not have an optical system. Transrectal probes have been used for imaging the prostate, bladder, uterus, and rectum. These probes are either linear array or mechanical sector.
ENDOSONOGRAPHIC ASSESSMENT OF GI MOTILITY AND BIOMECHANICS
In recent years ultrasound has been applied in studies of the biomechanical function of the GI tract. In this sense biomechanics should be understood in a much broader sense than the term motility. Motility considers active properties such as peristaltic contractions whereas biomechanics deals with active and passive forces and deformation of the tissue. Biomechanical alterations and abnormal motility in the GI tract is found in several diseases which may be localized in the organ itself or related to the nearest surroundings or to systemic diseases. Luminal manometry has so far been the most widely used method to study GI tract physiology. However, despite the development of high-resolution manometry systems there are still problems in the investigation of GI motor function. The strength of a contraction may vary considerably, thus giving more or less reliable manometric registrations. Furthermore, respiration, aortic pulsation and cardiac contractions may affect the recordings.
The presence of probes in the GI lumen has an influence on physiology and biomechanical studies should therefore ideally be examined without intraluminal devices. ES miniprobes combine the advantages of high ultrasound frequencies with small transducers, allowing detailed images of GI wall layers to be obtained with less influence on the GI lumen than the larger echoendoscopes. Hence, these probes have been regarded suitable for motility studies of the GI tract, especially when applied in lumina with small diameter, like the esophagus (Figure 2). However, echoendoscopes can also be used for examination of motility if positioned in wider lumina like the stomach. Since echoendoscopes generally utilize lower ultrasound frequencies and larger ultrasound probes they can also be used to study motility of neighbouring GI structures (Figure 3). Endosonographic real time B-mode imaging alone or in combination with other methods, as manometry and electromyography, have been used for studying correlation between the pressure and electric potential changes and structural changes in the GI wall. Correct interpretation of ultrasound images of the GI wall is crucial when applying this technique for detailed examination of physiological characteristics. The GI wall consists of mucosa, submucosa, muscularis propria and adventitia/serosa. Muscularis propria has an inner circular and an outer longitudinal layer that is separated by the myenteric plexus.
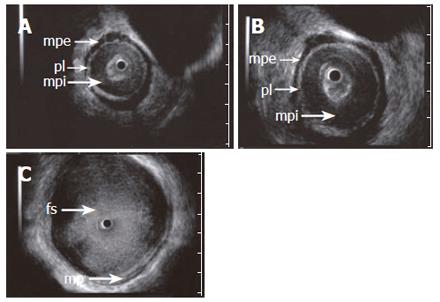
Figure 2 ES images (A and B) obtained with a 15 MHz miniprobe in a patient with achalasia showing the longitudinal, outer layer of the proper muscle (mpe) separated from a thickened circular, inner layer (mpi) by an echogenic layer corresponding to the localization of the plexus myentericus (pl).
C: shows a dilated esophageal lumen with food content (fs). A normal aspect of the proper muscle is seen.
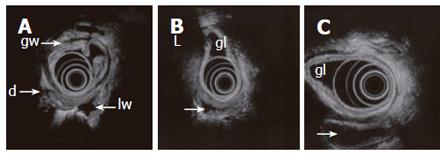
Figure 3 Endosonography with an echoendoscope (12 MHz frequency is used) placed in the gastric lumen (gl) showing the layers of the gastric wall (gw) and GI loops outside the stomach.
Lumen is partly collapsed (d), partly waterfilled (arrows). Motility and contractions of native GI loops may be studied. High frequency miniprobes are less suitable for this purpose due to higher frequencies and small transducer diameter.
The normal GI wall is imaged by ultrasonography as a layered structure consisting of 3-9 layers depending on the thickness and structure of different layers, ultrasound frequency, and applied pressure on the wall[6,7]. In most cases, a five-layer structure of the wall can be demonstrated, as determined by the histological layers and the appearance of interface echoes (Figure 2, Figure 4). When the visceral wall is imaged with an endoluminal transducer, the interface between the luminal contents and the mucosa accounts for the first, echo-rich layer. The rest of mucosa appears as a second, echo-poor layer. The third, echo-rich layer usually includes the interface between the lamina propria and muscularis mucosae, the submucosa, and the interface at the border of the muscularis propria. In a five-layer structure, the rest of muscularis propria is imaged as an echo-poor fourth layer. The fifth, echogenic layer represents the outer wall delineation and is an interface echo at the serosa or adventitia level.
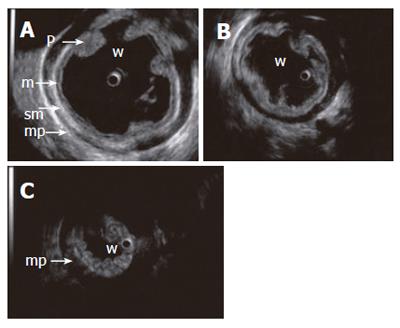
Figure 4 Contraction sequence (A, B, C) demonstrated with a 15 MHz miniprobe in a water-filled (w) stomach.
Gastric fold (p), mucosa (m), submucosa (sm), proper muscle (mp).
Sometimes, the use of high-frequency ultrasound produces an interface echo between the different aspects of the muscularis propria, probably reflecting the connective tissue embedding the myenteric plexus. A thick muscularis mucosa may also be identified as an echo-poor layer dividing the third, echogenic layer. Hence, the ultrasonographic wall structure may consist of seven or even nine different layers[6,8].
CLINICAL APPLICATIONS OF ENDOSONOGRAPHY IN BIOMECHANICS AND MOTILITY
Esophagus is often associated with motility disorders which can be studied with different techniques. Endosonography has been regarded as a suitable tool for examination of esophagus because this organ is a straight tube with a small diameter. McCray et al[9] studied the high pressure zone between the esophagus and stomach (lower esophageal sphincter and extrinsic crural diaphragm) in normal subjects with a dual high resolution endosonography-manometry catheter. This catheter recorded simultaneously manometric pressures and corresponding ultrasound images while being pulled through the distal esophagus. They found that simultaneous high-resolution endoluminal sonography and pressure manometry separate the two components of the high-pressure zone in the distal esophagus.
Endoscopic ultrasound findings have also suggested that an association exists between sustained longitudinal layer contraction in the esophageal body and chest pain of unknown origin[10]. Furthermore, Nicosia et al[11] combined manometry and ultrasound and used conservation of mass principles to quantify local axial shortening of the esophagus. A relationship between contractions in the circular and longitudinal muscle layers during swallowing was established. They also made 3D (space-time) reconstructions of the inner wall of the circular muscle layer for representative swallows. Takeda et al[12] used combined ultrasound and manometry to determine circumferential stress-strain properties in the esophagus. In a study by Taniguchi et al[13] the sheep esophagus was investigated with M-mode echoesophagram recording and manometry with the goal to measure simultaneously changes in the thickness of the individual esophageal wall layers and the corresponding changes in intraluminal pressure. The maximum manometric pressure, the esophageal wall layer thickness, and the duration of contraction were measured. Swallowing events up to one hour were recorded and in one trial both dry and wet swallows were compared. All swallowing events produced simultaneous increase in intraluminal pressure and esophageal wall thickness. The thickness of the inner (circular) muscle layer increased in dry as well as wet swallows.
The combination of manometry with a 20 MHz ultrasound transducer has also been used to correlate wall thickness, luminal diameter and contractive activity in healthy volunteers[14]. The increase in muscle width by ultrasonography was found to correlate with the increase in luminal pressure by manometry. Recently, Hoff et al[15] described a multimodal device inserted into esophagus combining bag distension, manometry, high frequency intraluminal ultrasound, laser Doppler flowmetry and symptom registration. Preliminary experience indicates that these modalities can be used in combination without significant technical influence on each other. Thus, this method can provide a basis for further studies, probably within both the upper and lower GI tract.
Achalasia
Achalasia is a disorder in esophageal motility and is defined by its typical pattern at manometry. The diagnosis can be supported by findings at barium radiography that may show delayed passage through the lower esophageal sphincter and dilatation of the body of the esophagus. At manometry, achalasia is characterized primarily by aperistalsis of the esophageal body and incomplete relaxation of the lower esophageal sphincter. In autopsy studies of patients with achalasia, the thickness of the lower esophageal sphincter is increased. Using high frequency US probes, it has been shown that the thickening of muscularis propria mainly appear in the inner circular portion (Figure 2). Thickening of the circular layer of the muscularis propria at the cardia may support the diagnosis of evolving achalasia[16-23].
A study of the esophageal wall in patients with newly diagnosed achalasia has also been performed using a sector scanning echoendoscope with 12 MHz transducer frequency. The endosonographic findings were correlated with manometric features and clinical symptoms. In these patients, an increase in total wall thickness and thickness of the muscularis propria was found at the level of the lower esophageal sphincter and the proximal 5 cm of the sphincter[16]. Miller et al[21] used a 20 MHz radial ultrasound transducer to examine the width of the total muscularis propria, and the circular and longitudinal smooth muscles at the lower esophageal sphincter in 29 patients with achalasia, comparing them with 19 normal subjects. They found that the mean width of these muscles was increased in patients with achalasia. However, because of overlap in muscle width thicknesses between these groups it was concluded that a thickened muscularis propria cannot be used to differentiate clinically between patients with achalasia and normal controls. Nevertheless, they found that the mean diameter of both the longitudinal and the circular smooth muscle layers at the lower esophageal sphincter were wider in patients with achalasia than in normal subjects. Trowers et al[22] used a single crystal transendoscopic probe providing linear images. Six achalasia patients were examined, demonstrating the thickness of the muscularis propria to be increased four-fold, and all patients were abnormal compared to controls.
Scleroderma
Patients suffering from systemic sclerosis have frequently clinical gastrointestinal manifestations, especially excessive deposition of collagen in the esophagus which may cause swallowing problems. Endoluminal US seems capable of defining morphologic as well as physiologic abnormalities of the esophagus in patients with scleroderma of the esophagus. Lieu et al[24] examined four patients and found that US images correlated with established pathologic descriptions like smooth muscle atrophy, variable fibrosis, and collagen deposition. Increased echogenicity and thinning of the muscularis propria, consistent with fibrosis and atrophy of the smooth muscle were observed. Miller et al[25] used a 20 MHz ultrasound transducer in examining 13 patients suffering from systemic sclerosis and 13 healthy subjects. Also postmortem autopsy study was performed to compare histopathological abnormalities with sonographic findings in the esophagus. In the postmortem ultrasound study of two cases, hyperechoic echoes were seen in the normally hypoechoic muscularis propria. These findings corresponded to fibrosis found in histological examinations. Hyperechoic abnormalities were ultrasonographically seen in the muscularis propria in patients but not in normal controls. A strong positive correlation between esophageal hyperechoic and manometric abnormalities was also found.
Nutcracker esophagus
Patients with nutcracker esophagus (NE) suffer dysphagia and/or chest pain due to strong peristaltic contractions. High-pressure amplitudes are found at manometry. Melzer et al[26,27] used an echoendoscope to examine the thickness of the muscularis propria at the gastroesophageal junction and in the lower, middle, and upper esophagus in patients with nutcracker esophagus. The muscularis propria was thickened in one-third of the patients. This thickening, however, did not correspond to the location and magnitude of the manometric abnormality and did not correlate with the clinical presentation. They also reported a patient with NE and esophageal pressures exceeding 800 mmHg in whom endoscopic ultrasonography demonstrated a markedly thickened esophageal muscularis propria. The hypertrophy of the muscularis propria extended from the gastroesophageal junction upwards to the middle esophagus.
Reflux esophagitis
Esophageal dysfunction can be seen in patients with reflux esophagitis. Important pathophysiological factors are impaired esophageal acid clearance and a hypotonic lower esophageal sphincter. The intraluminal esophageal pressure is related to the contraction of the inner circular smooth muscle. Dysfunction of the lower esophageal sphincter area may cause esophageal reflux disease. Contraction of the longitudinal smooth muscle during swallowing causes axial shortening of the esophagus. This muscle may also stiffen the esophagus and thus generate intraluminal pressure during peristalsis. In patients with reflux esophagitis, inflammatory changes are present, affecting all or individual wall layers in the distal part of the esophagus. Periesophageal inflammation may also be present and, depending on the degree of total inflammation, it may affect the esophageal motility and thus also acid clearance. These changes can usually be imaged by ultrasound miniprobes, which thus may provide insights into the pathophysiology of reflux esophagitis.
GASTRODUODENAL MOTILITY
Endosonography of the stomach and duodenum is in essence performed in the same way as in the esophagus. However, endosonography can be technically more challenging in wide and curved organs. Transabdominal ultrasound is applied in studying gastroduodenal motility, also in combination with intraluminal pressure devices. Recently, mechanosensory properties in the human gastric antrum were evaluated using transabdominal ultrasound scanning during volume-controlled antral distension[28,29]. Furthermore, a device which combines endosonographic real - time imaging with intraluminal volume-controlled bag distensions has been developed also for use in large lumina. Thus, detailed images of GI wall layers corresponding to the area of bag pressure can be obtained. Arslan et al[30] investigated duodenum with high-frequency ultrasound to study wall layers and motility during allergenic provocation. A 20 MHz miniature ultrasound probe was inserted through a nasoduodenal tube and ultrasound examination was performed before and after provocation. Clinical symptoms were recorded and the thickness of mucosa, submucosa and muscularis propria was measured directly on ultrasonographic images. Endosonographic changes (i.e. any resolvable wall thickening, contraction or new echogenic layer) were observed in 15 of the 20 patients. Mucosal thickening, changes in the number of wall layers and sustained contractions of duodenum were observed in some patients after provocation. The mucosal thickening could represent mucosal edema in response to provocation. A new echorich layer was observed in two patients, probably caused by an additional interface at the border of a thicker muscularis mucosa due to the allergic reaction.
Rectum, large and small intestine
These organs may be examined in the same way as described for esophagus, stomach and duodenum using dedicated echoendoscopes and rectal probes. However, ES miniprobes, which can easily be inserted through the biopsy channel of standard endoscopes, seem to be a practical method for imaging the colon as well as the rectum. The interpretation of wall layers, organ contractions and other motility aspects is similar to that of the upper GI tract. Endoscopy of the small intestine is now possible in some GI laboratories. New enteroscopes may in the future be integrated with ultrasound transducers making ES/EUS of the small intestine possible. Capsule endoscopy which also is a relatively new method, allows endoscopic imaging of the entire small intestine. If a miniature ultrasound device is integrated in this capsule, EUS of the small intestine may possibly be performed.
NEW TECHNIQUES
Three-dimensional EUS
3D- images are assumed to be easier to understand and communicate than the mental reconstruction of several 2D-images. 3D-techniques are currently under development for most imaging modalities and 3D-EUS may be applied for improved recognition of the GI anatomy and pathological lesions. 3D-EUS images can be obtained using images acquired by echoendoscopes or miniprobes. Mostly, a pullback device has been used to obtain parallel 2D images which are reconstructed to a 3D-image. Acquired ultrasound images can be stored and digitized using frame grabbing but direct digitizing of ultrasound raw data is being increasingly used in new scanners. Advanced software programs containing different rendering or other reconstruction algorithms are used for postprocessing and display of 3D ultrasound data, which can be studied using anyplane slicing, segmentation and volume calculation[31,32].
Elastography
The stiffness of normal and pathological tissue is usually different and may be evaluated by ultrasound which is based on B-mode scanning during compressions (elastography) (Figure 5). Elastography has been applied on the GI tract, kidney, muscle, breast and the heart. The underlying principle is that the deformation of tissue by a mechanical excitation is a function of its mechanical properties. For gastrointestinal purposes, the intraluminal pressure may be used as the excitation force[33,34].
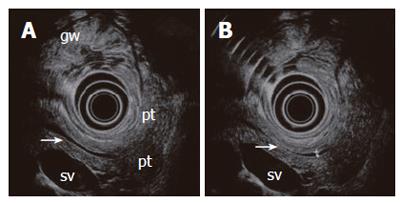
Figure 5 A normal pancreas imaged from the stomach with a 6 MHz echoendoscope.
The compressed posterior gastric wall is closely related to the surface of the pancreas. The pancreatic duct is seen (arrow) with minor (A) and slightly increased transducer pressure (B). The non-compressed gastric wall with folds (gw) is seen on the opposite side of the transducer. Pancreatic tail (pt), splenic vein (sv).
Imaging of ultrasound tissue elasticity has gained considerable interest as a method to differentiate normal from abnormal tissue, particularly malignant from non-malignant disease. However, normal structures also have variable internal sonoelastic properties. When compressed by an ultrasound transducer, the ultrasound image of normal as well as pathological tissue is changed. It has been demonstrated that increased pressure on the GI wall changes the thickness, layers and echogenicity of the wall as imaged by B-mode ultrasonography[7].
Strain rate imaging
Strain rate imaging is a recently introduced technique to measure deformation of biological tissue. The technique is based on tissue velocity imaging, which is an ultrasound technique that provides quantitative information on velocities within a tissue. By color-coded tissue velocity imaging, velocity data from the whole field of view are available simultaneously[35]. This allows extraction of other parameters through spatial and temporal processing of the velocity data. Strain rate and strain are examples of such parameters. The methods have primarily been used in echocardiography, but other uses have also been reported, including measurements of gastric motor function[36].
Further development of specialized EUS instruments can allow the investigators to choose the most appropriate instruments for the separate tasks. Miniature probes, which are less costly and easier to operate, will most likely be available in a majority of endoscopy units within a few years. Different modalities can probably be merged into new multimodal devices making it possible to obtain multiple motility parameters in one procedure. A successful development of such devices can hopefully be achieved on the basis of fruitful collaboration between medicine, technology and soft-ware producers.
S- Editor Pan BR E- Editor Bai SH