INTRODUCTION
Platelet-activating factor (PAF) is a potent pro-inflammatory lipid mediator that sparks a wide range of immunoregulatory actions[1]. A phospholipase A2 (PLA2)-dependent process on membrane alkyl-acyl-glycerophosphocholines generates the lyso-PAF precursor, and its subsequent acetylation results in the PAF molecule[2]. Tissue PAF concentrations are physiologically down-regulated by a specific PAF acetylhydrolase activity (AHA)[2]. PAF acts through specific PAF-receptors (PAF-R) present on the membrane of responsive cells[3]. Several experimental animal models have highlighted that the liver PAF content was elevated by various types of injury, including endotoxin exposure[4,5], ischemia-reperfusion[6], hemorrhagic shock[7], and carbon tetrachloride (CCl4)-induced cirrhosis[8,9]; results of all these animal studies leading to the conclusions that PAF is a potent vasoconstrictor and systemic vasodilator. While animal models have reported the involvement of PAF in experimental cirrhosis, no clinical study has directly investigated its values in the human cirrhosis liver.
Several in vitro studies have highlighted the potential involvement of PAF in carcinogenesis. Thus, PAF acts on the growth of various human tumor cell lines [10,11], increases adhesiveness of tumor cells to vascular endothelia[12], enhances oncogene expression[13], and can contribute to tumor development by enhancing cell motility and by stimulating the angiogenic response[14,15]. Clinical studies have reported elevated PAF amounts in human breast[16], and colon carcinoma[17,18]. However, PAF was not found in higher amounts in lung[19], and thyroid carcinoma[20], suggesting that over-expression of PAF is not necessarily a universal event in carcinogenesis but may be tumor-specific. At present, to our knowledge, no study has investigated PAF levels in hepatocellular carcinoma (HCC). HCC is a hypervascular tumor. Several angiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and angiopoietins, have been reported to promote angiogenesis in HCC[21]. Of interest, PAF is known to mediate the effect of these angiogenic growth factors in several in vitro and in vivo models[22-24], suggesting a putative involvement of PAF in HCC.
To improve our knowledge concerning the role of PAF in the human liver, we investigated the levels of PAF and lyso-PAF, the enzymatic activities implicated in PAF production (i.e., PLA2) and degradation (i.e., AHA) and the presence of PAF-R transcripts in human cirrhotic and HCC tissues.
MATERIALS AND METHODS
Subjects
The procedure of the present study followed the general rules edited by the French National Ethics. Between January 1999 and December 2004, 29 consecutive patients (27 men and 2 females; mean age 62.5 years (range 30-78 years), median age 68 years) with HCC underwent resection in the Department of Surgery of Limoges’ CHU. Resectability was preoperatively assessed by ultrasonography, computed tomography and/or magnetic resonance imaging. Intraoperative ultrasonography was performed in all patients. The operative procedures included 4 wedge resections, 10 segmentectomies, 7 left hepatectomies, 6 right hepatectomies and 2 total hepatectomies before liver transplantations. A curative resection, defined as an operation in which all the tumors were macroscopically resected during surgery, was performed in 21 patients (72%). The mean margin from the edge of the tumor was 7.5 ± 1.5 mm. The mean and median tumor size was 7.79 ± 4.6 cm and 8.2 cm, respectively. Twenty tumors (69%) were solitary. Cirrhosis was present in 14 patients (48%), with the cause being alcoholism in 46%, hepatitis C in 35%, hepatitis B in 5%, alcohol and hepatitis in 7%, and unknown in 7%. Two patients had liver fibrosis without cirrhosis, 6 had steatosis, and 7 had no liver disease. Histologically, there were 10 well differentiated HCC (34%), 14 moderately differentiated HCC (48%), 4 poorly differentiated HCC (14%), and 1 fibrolamellar carcinoma, according the histopathologic criteria cited by Kojiro M[25]. Thirteen HCC (44%) had a fibrous capsule that was invaded in 68%. Vascular invasion was present in 19 cases (65%) and 8 HCC presented a high mitosis index. According to pT staging[26], there were 2 pT1, 8 pT2, 13 pT3 and 6 pT4 HCCs. Specimens for the pathologic tissue and the control tissue close to the pathologic one were obtained during the surgical procedure. Specimens were frozen at -80 °C until used.
Platelet-activating factor assay
Tissue samples were ethanol-extracted (80% final), purified using thin layer chromatography (TLC), and assayed for PAF activity by aggregation of washed rabbit platelets as previously reported[17-20]. The aggregating activity of samples was measured using a calibration curve obtained with 2.5 to 20 pg of synthetic PAF (Novabiochem, Switzerland). Results were expressed as picograms of PAF per mg of tissue. The lipid compound extracted from blood was further characterised on the basis of its aggregating activity in the presence of 0.1 mmol/L BN 52021 (Tebu, Le Perray-en-Yvelines, France), a specific PAF receptor antagonist and its retention time during TLC.
Assay of lyso-PAF
Lyso-PAF was measured in ethanolic biopsy sample after its chemical acetylation into PAF as previously described[17-20]. Briefly, ethanolic samples were dried, mixed with 200 μL of pyridine and 200 μL of acetic anhydride, and kept overnight in the dark at room temperature. After evaporation of the reagents and removing of the traces of pyridine with chloroform, the dried samples were retrieved with 100 μL of 600 mL/L ethanol, and PAF was bioassayed as described before. The amount of lyso-PAF was established as the difference between the quantity of PAF measured before and after acetylation of the samples. Results were expressed as picograms of PAF per mg of tissue.
Acetylhydrolase assay
Frozen biopsy specimens were pulverised and homogenised in 1 mL of AHA buffer (140 mmol/L NaCl, 3 mmol/L KCl, 4 mmol/L HEPES, 22 mmol/L EDTA). After centrifugation, supernatants were used for AHA and PLA2 determinations. AHA was assessed as previously described[17-20]. Briefly, 105 dpm of [3H]acetyl-PAF (10 Ci/mmol, NEN), 0.1 mmol/L PAF, AHA buffer (pH 8) in a final volume of 450 μL, and 50 μL of tissue extract supernatants were incubated for 30 min at 37 °C. The reaction was stopped with addition of 100 μL of bovine serum albumin (100 g/L) and 400 μL of trichloracetic acid (200 g/L). Samples were centrifuged at 1500 g for 15 min, and supernatants were counted in a liquid scintillation counter. Results were expressed as fentomoles of PAF degraded per min per mg of tissue as means of duplicate assays. Variation between duplicates was less than 7%.
PLA2 activity assay
PLA2 levels were assessed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems Europe, Oxon, UK) and as previously described[17,18,20]. Results were expressed as international unit (IU) per mg of tissue as means of duplicate assays. Variation between duplicates was less than 6%
Reverse-transcriptase polymerase chain reaction (RT-PCR) of PAF-R transcripts
Total RNA from tissue samples extracted with RNAwiz (Ambion, Austin, TX) was reverse-transcribed and the complementary DNA (cDNA) was amplified by PCR as previously described[17,18,20]. The human PAF-R transcript 1 sense primer was 5’-GACAGCATAGAGGCTGAGGC-3’, the transcript 2 sense primer was 5’-CCTGAGCTCCCCGAGAAGTCA-3’ and the antisense primer was 5’-TAGCCATTAGCAATGACCCC-3. The sense and anti-sense primers of glyceraldehyde-3-phosphate dehydrogenase (GADPH, a positive control of PCR amplification) were 5’-GGCTGAGAACGGGAAGCTTG-3’ and 5’-GGATGATGTTCTGGAGAGCC-3’, respectively. PCR products were electrophoresed on a 20 g/L agarose gel (Gibco, France) and visualised by ethidium bromide staining. Expected sizes of amplified products were: PAF-R transcript 1225 bp; PAF-R transcript 2269 bp; spliced variant of PAF-R transcript 2351 bp; and GAPDG, 439 bp.
Statistical analysis
Differences between groups were assessed using the Mann-Whitney U-test. A paired student’s t-test was used to analyse intragroup differences. P < 0.05 was considered statistically significant.
RESULTS
PAF and human cirrhosis
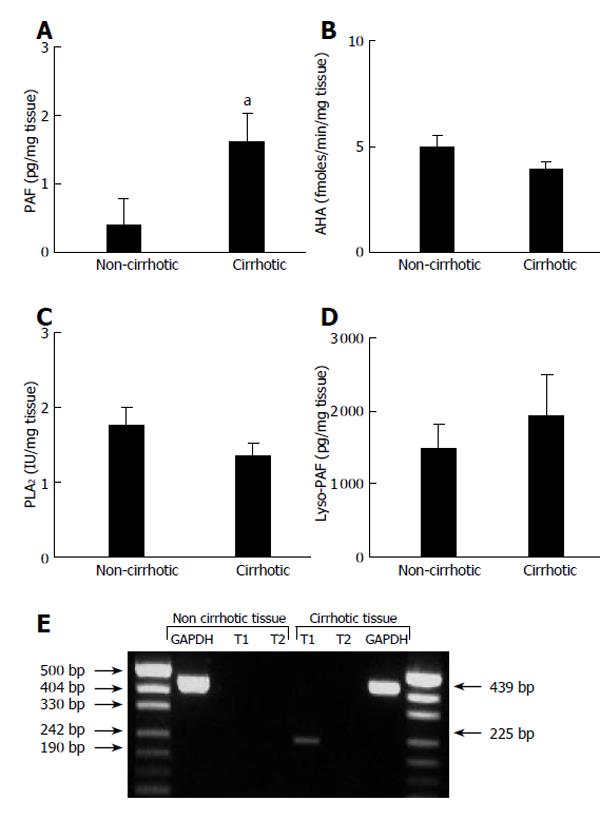
A 4-fold increase of PAF levels was found in the cirrhotic tissue (1.61 ± 0.43 pg/mg, n = 14) as compared to the non-cirrhotic one (0.39 ± 0.39 pg/mg, n = 7) (Figure 1A). In contrast, the AHA levels (the PAF degrading enzyme) were unchanged (P = 0.33, Mann-Whitney U-test) in the cirrhotic tissue (3.93 ± 0.38 fmol/(min.mg), n = 14) as compared to the non-cirrhotic tissue (5.00 ± 0.53 fmol/(min.mg), n = 7) (Figure 1B). Similarly, PLA2 levels (the enzymatic activity that generated the lyso-PAF precursor) were unchanged (P = 0.23, Mann-Whitney U-test) in the cirrhotic tissue (1.35 ± 0.17 U/mg, n = 14) as compared with non-cirrhotic one (1.76 ± 0.24 U/mg, n = 7) (Figure 1C). In agreement with these latter results, amounts of the lyso-PAF were similar (P = 1, Mann-Whitney U-test) in the cirrhotic tissue (1 934.0 ± 556.9 pg/mg, n = 14) as compared to the non-cirrhotic one (1 488.9 ± 331.0 pg/mg, n = 7) (Figure 1D).
Figure 1 PAF, lyso-PAF, AHA, PLA2 levels and PAF-R transcripts in cirrhotic and non-cirrhotic liver tissues.
Upper panel shows the specimens of cirrhotic (n = 14) and non-cirrhotic tissue (n = 7) obtained during the surgical procedure. A: PAF determined by a platelet-aggregation assay, aP<0.05 vs non-cirrhotic; B: AHA determined by investigating the degradation of [3H]-PAF, (P = 0.33); C: PLA2 assessed by enzyme-linked immunosorbent assay, (P = 0.23); D: Lyso-PAF measured after its chemical acetylation into PAF, (P = 1.00). Results are expressed as mean±SE. Statistical analysis was performed using the Mann Whitney U-test. Lower panel shows PAF-R transcripts determined by RT-PCR. Expected sizes of amplified products were 225 bp (PAF-R transcript 1), 269 bp (PAF-R transcript 2), 351 bp (spliced variant of PAF-R transcript 2), and 439 bp (GAPDG). Sizes of PCR products and DNA size ladder are indicated by arrows. All experiments were performed in triplicate.
PAF-R and human cirrhosis
The PAF-R gene produces three different species of mRNA [i.e., transcript 1 (leucocyte-type), transcript 2 (tissue-type) and an elongated form of the transcript 2]; both transcripts ultimately yield the functional PAF-R. As shown in Figure 1 (lower panel), RT-PCR experiments showed the presence of PAF-R transcript 1 but not transcript 2 in the cirrhotic tissue.
PAF and human HCC
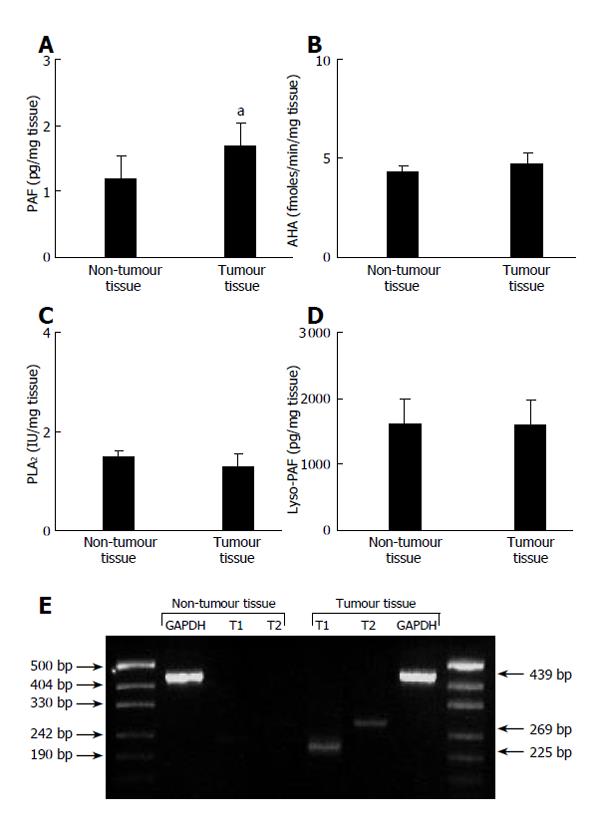
A significant (P = 0.0002, t-test for paired data) increase of PAF levels was found in tumor tissues (1.69 ± 0.34 pg/mg, n = 21) as compared to the non-tumor ones (1.20 ± 0.33 pg/mg, n = 21) (Figure 2A). This increase was significant (P = 0.0044, student t-test for paired data) for cirrhotic patients (1.88 ± 0.41 pg/mg vs 1.61 ± 0.43 pg/mg for tumor and non-tumor tissues, respectively) and also (P < 0.05, student t-test for paired data) for non-cirrhotic patients (1.29 ± 0.62 pg/mg vs 0.39 ± 0.30 pg/mg for tumor and non-tumor tissues, respectively). For HCC patients, PAF values were not significantly different (P = 0.52, Mann-Whitney U test) according to the tumor status (1.85 ± 0.49 pg/mg vs 1.62 ± 0.44 pg/mg for six T1/T2 patients and fifteen T3/T4 patients, respectively). Only tumor PAF levels were affected in HCC tissue. Indeed, the enzymatic activities implicated in PAF production and degradation were not changed. Thus, the AHA levels (the PAF degrading enzyme) were unchanged (P = 0.24, t-test for paired data) in the tumor tissue (4.72 ± 0.52 fmol/(min.mg), n = 21) as compared to the adjacent tissue (4.29 ± 0.32 fmol/(min.mg), n = 21) (Figure 1B). Amounts of the lyso-PAF were unchanged (P = 0.48, t-test for paired data) in the tumor tissue (1 588.9 ± 377.9 pg/mg, n = 21) as compared to the non-tumor one (1 616.8 ± 373.7 pg/mg, n = 21) (Figure 1D). Moreover, PLA2 levels (the enzymatic activity that generated the lyso-PAF) were unchanged (P = 0.26, t-test for paired data) in HCC tissue (1.28 ± 0.27 U/mg, n = 21) as compared with non-tumor one (1.49 ± 0.14 U/mg, n = 21) (Figure 1C).
Figure 2 PAF, lyso-PAF, AHA, PLA2 levels and PAF-R transcripts in HCC and non-tumour tissues.
Upper panel shows specimens from 21 HCC patients obtained during the surgical procedure. A: PAF, aP < 0.05 vs non-tumour tissue; B: AHA (P = 0.24); C: PLA2 (P = 0.26); D: Lyso-PAF (P = 0.48). Statistical analysis was performed using the Students’ t-test for paired samples. Results are expressed as mean ± SE. Lower panel shows PAF-R transcripts determined by RT-PCR. Expected sizes of amplified products were 225 bp (PAF-R transcript 1), 269 bp (PAF-R transcript 2), 351 bp (spliced variant of PAF-R transcript 2), and 439 bp (GAPDG). Sizes of PCR products and DNA size ladder are indicated by arrows. All experiments were performed in triplicate.
PAF-R and human HCC
As shown in Figure 2 (lower panel), RT-PCR experiments indicated the presence of PAF-R transcripts 1 and 2 in HCC tissues. The spliced variant of PAF-R transcript 2 was not detected.
DISCUSSION
Elevated PAF levels were found in the cirrhotic tissue as compared to the non-cirrhotic one, confirming, for the first time in human, the results obtained in experimental models of liver cirrhosis[8,9]. Elevated levels of PAF can result from a higher PAF production or a lower PAF degradation. Arguing against the latter hypothesis, the AHA levels (the PAF degrading enzyme) were unchanged in the cirrhotic tissue as compared to the non-cirrhotic tissue. Thus, the elevated PAF levels in cirrhotic tissue might be due to an elevated PAF synthesis. Kupffer cells which are a well known source of PAF[27] might be an appropriate candidate for these elevated tissue PAF levels. Strengthening this hypothesis, Kupffer cells from cirrhotic rat was found to produce more PAF than kupffer cells of control rats[8]. Confirming a previous study[28], PLA2 levels (the enzymatic activity that generated the lyso-PAF) were unchanged in the cirrhotic tissue. In agreement with these latter results, amounts of the lyso-PAF were similar in the cirrhotic tissue as compared to the non-cirrhotic one. PAF acts as a local cell-to-cell mediator. Thus, an elevated PAF synthesis is only of relevance if PAF-R can be detected at the site of PAF production. The PAF-R gene produces three different species of mRNA (i.e., transcript 1, transcript 2 and an elongated form of the transcript 2); both transcripts ultimately yield the functional PAF-R[3]. Leucocytes produce PAF-R transcripts 1, while PAF-R transcripts 2 are found in organ extracts. Thus, PAF-R transcripts 1 and 2 are also named “leukocyte-type” and “tissue-type”, respectively. RT-PCR experiments indicated the presence of PAF-R transcript 1 in the cirrhotic tissue. These PAF-R transcripts from the “leukocyte-type” fit well with the previous study that showed higher hepatic levels of PAF-R mRNA in CCl4-induced liver cirrhosis[8], with cirrhotic kupffer cells as cellular source of these PAF-R mRNA. Thus our present study concludes that PAF and PAF-R transcripts are present in the human cirrhotic liver. PAF may have pathophysiologic functions and might account, at least in part, in the inflammatory response occurring in the cirrhotic liver. On the one hand, PAF might act directly by stimulating the production of humoral mediators including peptide leukotrienes (LTC4, LTD4, LTE4)[29], prostaglandin E2 (PGE2)[30], and nitric oxide[31], which can affect cell proliferation and gene expression. On the other hand, and with respect to its potent immunoregulatory actions[1,32], PAF might act indirectly on stellate cell-immune cell interactions by promoting leukocyte chemotaxis and adherence, by influencing leukocyte activation and by affecting their cytokine secretions.
Previous clinical studies have reported the elevated PAF levels in breast and colon carcinomas[16-18], but not in lung and thyroid carcinomas[19,20], suggesting that over-expression of PAF is not necessarily a universal event in carcinogenesis, but may be tumor-specific. Increased PAF levels were found in HCC tissues. In contrast to colorectal cancer patients[17], tumor PAF level did not change according to the tumor status. As suggested above, the elevated PAF levels in HCC tissue might be due to elevated PAF synthesis by Kupffer cells. In contrast to that found in colon carcinoma[17], only tumor PAF levels were affected in HCC tissue. Indeed, the enzymatic activities implicated in PAF degradation (i.e., AHA) and production (i.e., PLA2) were not changed; these latter results fitting well with the data reporting no differences in phospholipids composition (including phosphatidylcholine one) between human hepatoma homogenates and normal regions outside the tumors[33]. Few clinical studies had focussed on PAF-R levels in cancer tissue. Levels of PAF-R transcripts were not modified in colorectal, lung and thyroid carcinoma[17,19,20], while PAF-R (tissue-type) levels were increased in liver metastasis of colorectal cancer[18]. PAF-R transcripts 1 (leukocyte-type) and 2 (tissue-type) were found in the HCC tissues. Kupffer cells might be the cell source for the elevated levels of PAF-R (leukocyte-type) transcripts. Thus, transforming growth factor beta (TGF-β) and PAF stimulated PAF-R mRNA synthesis in monocytic/macrophagic cells[34,35]. Elevated levels of TGFβ[36] and PAF (Figure 2A) are found in HCC tissues. PAF-R transcript 2 (tissue-type) has also been reported to be up-regulated in HCC tissue. Such an up-regulation of PAF-R transcripts 2 has been previously reported in endometrial cells in response to estradiol[37]. Interestingly, locally elevated estrogen formation has been reported in HCC tissues[38], and estrogen enhances angiogenesis through a pathway involving PAF in a mouse Matrigel model in vivo[39]. Thus, whatever molecular signals implicated in these elevated PAF-R transcript levels, the current data argue for a pathophysiological role of PAF in HCC tissues.
In conclusion, PAF is a potent pro-inflammatory compound that acts as a local cell-to-cell mediator. Since PAF can generate biological responses detectable at levels of 10 fmol/L, regulating PAF levels is important since elevated levels of PAF might result in biological effects. To the best of our knowledge, this clinical study, for the first time, reports an increase in PAF and PAF-R transcripts in cirrhotic liver tissue and HCC tissue, suggesting a pathological role for PAF in liver cirrhosis and HCC. In cirrhotic tissue, PAF might act on stellate cell-immune cell interactions by influencing leukocyte activation and chemotaxis, and by altering the local cytokine network. An angiogenic role of PAF might be suggested in HCC.