Published online May 7, 2006. doi: 10.3748/wjg.v12.i17.2737
Revised: October 1, 2005
Accepted: October 26, 2005
Published online: May 7, 2006
AIM: To investigate the interaction between portal hypertension, splanchnic hyperdynamic circulation and splanchnic vasculopathy by observing splenic arterial and venous pathological changes and the ro1e of extra-cellular matrix in the pathogenesis of portal hypertensive vasculopathy by measuring the expression of type Ι and type III procollagen mRNA in splenic venous walls of portal hypertensive patients.
METHODS: Morphological changes of splenic arteries and veins taken from portal hypertensive patients (n = 20) and normal controls (n = 10) were observed under optical and electron microscope. Total RNA was extracted and the expression of type Ι and type III procollagen mRNA in splenic venous walls of portal hypertensive patients (n = 20) was semi-quantitatively detected using reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS: Under optical microscope, splenic arterial intima was destroyed and internal elastic membrane and medial elastic fibers of the splenic arterial walls were degenerated and broken. Splenic venous intima became remarkably thick. Endothelia1 cells were not intact with formation of mural thrombus. The tunica media became thickened significantly due to hypertrophy of smooth muscles. Fibers and connective tissues were increased obviously. Under electron microscope, smooth muscle cells of the splenic arteries were degenerated and necrotized. Phenotypes of smooth muscle cells changed from constrictive into synthetic type. Red blood cells and platelets accumulated around the damaged endothelial cells. Synthetic smooth muscle cells were predominant in splenic veins and their cytoplasma had plentiful rough endoplasmic reticulum ribosomes and Golgi bodies. Along the vascular wall, a lot of collagen fibers were deposited, the intima was damaged and blood components accumulated. There was no significant difference in the expression of type I procollagen mRNA in splenic venous wall between the patients with portal hypertension and those without portal hypertension (P > 0.05), but the expression of type III procoagen mRNA was significantly stronger in the patients with portal hypertension than in those without portal hypertension (P < 0.01).
CONCLUSION: Type III procollagen and collagen might be important extra-cellular matrix resulting in neointimal formation and vascular remodeling in the pathogenesis of portal hypertensive vasculopathy. The pathological changes in splenic arteries and veins exist in portal hypertension patients. There might be an interaction between portal hypertension, splanchnic hyperdynamic circulation and splanchnic vasculopathy.
- Citation: Li T, Ni JY, Qi YW, Li HY, Zhang T, Yang Z. Splenic vasculopathy in portal hypertension patients. World J Gastroenterol 2006; 12(17): 2737-2741
- URL: https://www.wjgnet.com/1007-9327/full/v12/i17/2737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i17.2737
With the elevation of portal pressure, obvious pathological changes in splanchnic vessels occur, such as extensive formation of porto-systemic communicating branches, remodeling of splanchnic great arteries and veins, which are defined as portal hypertensive vasculopathy (PHV). PHV is compensation of splanchnic vessels for hyperdynamic circulation, but also aggravates the portal pressure. PHV is one of the direct causes of variceal hemorrhage[1,2]. In this study the splanchnic vessels in portal hypertensive patients were observed under optical and electron microscope in order to explore the interaction between portal hypertension, splanchnic hyperdynamic circulation and splanchnic vasculopathy. The expression of types I and III procollagen mRNA in the splenic venous wall was detected by RT-PCR in the patients with portal hypertension in order to explore their ro1e in the pathogenesis of portal hypertensive vasculopathy.
From June 2002 to October 2002, 20 patients including 15 males and 5 females with a mean age of 41 years with portal hypertension were selected from Center of Hepatic Surgery of Tongji Hospital and Caidian Schistosomiasis Prophylactic therapeutic Institution. Among them 14 belonged to Child class A and 6 to Child class B. Endoscopic examination revealed that all patients had moderate or serious gastroesophageal varices accompanied with hypersplenism and splenomegaly. Pathologically, there were 12 cases of schistosomial hepatic cirrhosis and 8 cases of post-hepatitis hepatic cirrhosis. Eight cases had a history of one or more episodes of massive upper digestive tract hemorrhage. Eight cases received splenectomy in combination with esophagogastric devascularization and twelve cases splenectomy. The control group I included 10 patients (6 males and 4 females with a mean age of 38 years) with traumatic spleen rupture without liver cirrhosis and all were subjected to emergency splenectomy. A piece of splenic arteries and veins near the hilus of the spleen was removed during the operation under laser scanning confocal microscope (Germany). PCR device (PTC-200 type) was the product of MJ Research Inc., (USA). UVP gel imaging analysis system was the product of UVP Company (England). M-MLV reverse transcriptionase, Oligo(dT)15 primer, DNTP, TaqNa polymerase were the products of Promega Company(USA). PCR marker was the product of Dalianbao Bioengineering Company.
The vascular specimens were fixed with 40 g/L neutrally buffered formaldehyde, routinely embedded with paraffin, cut into 5μm thick sections and stained with H&E. The sections were observed under microscope. The fresh vascular specimens were cut into 1mm x 1mm x 1mm senctions, put into 25 mg/L glutaraldehyde within 1 min for pre-fixation for 2h, washed with phosphate buffer (pH 7.4), post-fixed with 10g/L osmic acid at 4 °C for 2h, then dehydrated with ascending concentrations of alcohol and acetone, embedded with epoxy resin Epon812, and finally cut into ultrathin sections. The sections were double stained with plumbum and observed under a transmission electron microscope (Opton EM l0C Model). After dehydration with alcohol, the sections were dried at a critical point, sherardized in vacuum, then examined and photographed under a Nikon scanning electron microscope(S-520 model).
RT-PCR primers were designed according to Primer 5.0 version [human (a1) type І proco11agen(GenbankZ-74615): upstreamprimer5’-ACCGCAAACCTTTCTACTTC-3’ and downstream primer 5’-AAGGCGAGTAGGAGCAGT-3’, 465 bp; human (al) type III procollagen (GenbankX-14420): upstream primer 5’-ACCGCAAACCTTTCTACTTC-3’ anddownstreamprimer 5’AAGGCGAGTAGGAGCAGT-3’, 384 bp; human GAPDH (Genbankm 33197) served as house-keeping gene internal reference; upstream primer 5’-GGATTTGGTCGTATTGGG-3’and downstream primer 5’-GGAAGAGGTGAGGGATT-3’, 384 bp]. The total RNA from splenic veins was extracted by Trizol one-step method. In each sample containing 5 mg RNA, DEPC was added to a volume of 27 μL following addition of 15 μL (1 ug) o1igo (dT). After degeneration of RNA by incubation at 70 °C for 5 min, 5 × 8 μL buffer, 4 × 2 μL dNTP, 1 μL RNAase inhibitor, 2 μL MMLV (400U) were added. In a total vo1ume of 40 μL, reverse transcription was performed at 42 °C for 1h fo11owed by denaturation at 95 °C. A total volume of PCR mixture contained 10 × 5 μL Buffer, 3 μL 25 mmo1 MgCl2, 4 x 1 μL dNTP (10 pmol/mL), 5 μL upstream (10pmo1/L), 5 μL cDNA, 1 μL Taq enzyme (3 U), 33 μL DEPC. PCR conditions were 30 cycles of annealing at 52 °C for human type I and III pro collagen, 30 cycles of annealing at 51 °C for GAPDH. The PCR amplified products (10mL) were spotted and electrophoresed on 15 g/L gel at 5 V/cm. The results were observed under ultra-violet light. UVP gel imaging analysis system (England) was used to analyze the density of electrophoresis bands of PCR products. The targeting gene expression intensity = targeting gene RT-PCR product A÷GAPDH RT-PCR product A
All data were expressed as mean ± SD. Homogeneity test of variance, ANOVA and t test were performed by SPSSll.0 statistical software. P < 0.05 was considered statistically significant.
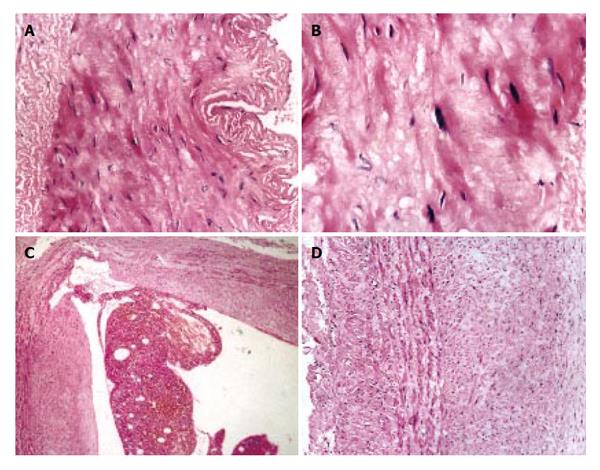
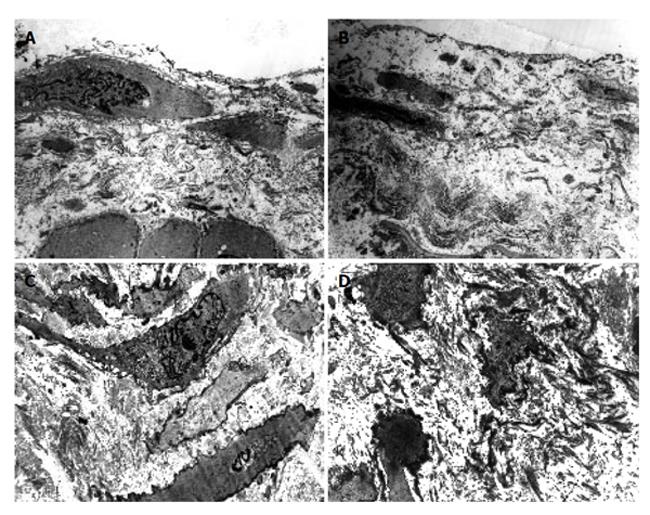
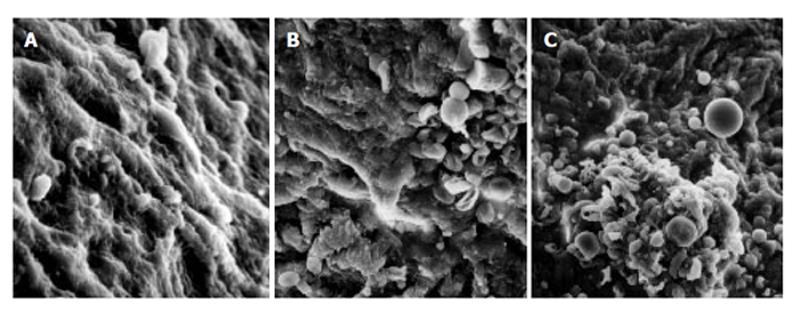
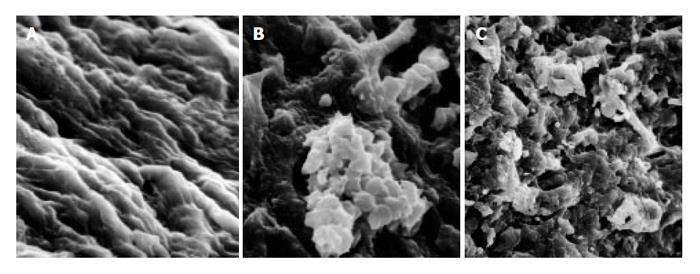
As compared with the patients without hepatic cirrhosis, spleen of the patients with hepatic cirrhosis was significantly enlarged, splenic arterial and venous walls were obviously thickened, vascular caliber was increased and elasticity was decreased. Under optical microscope, intima of the normal splenic arterial walls was smooth, endothelial cells were intact and no subintimal smooth muscle cells were seen. In the tunica media, multiple layers of smooth muscle cells were separated by collagenous and elastic fibers. In the patients with hepatic cirrhosis, intima of the splenic artery was significantly thickened, endothelial cells were not intact, internal elastic membrane was broken and smooth muscle cells in tunica media were disturbed (Figures 1A and 1B). Intima of the normal splenic veins was intact and endothelial cells were not destroyed. In the patients with hepatic cirrhosis, intima of the splenic vein was locally thickeried, endothelial cells were not intact, intima was damaged and mural thrombus formed (Figures 1C and 1D). Under electron transmission microscope, smooth muscle cells of the normal splenic artery were fusiform and nuclei were long-circular in shape. In cytoplasma, there were myofilaments, mitochondria, endoplasmic reticulum ribosomes, Golgi bodies and gluycogenic granula. In the patients with hepatic cirrhosis, endothelial tissue of the splenic artery was damaged, internal elastic membrane was broken and elastic fibers were ruptured. In the tunica media, smooth muscle cells increased and migrated into subintima. Partial smooth muscle cells had atrophy and degeneration. Local cytoplasma was necrotized and formed vacuoles. The number of mitochondria was increased, their cristae disappeared and vacuoles formed. Collagenous fibers along the vascular wall were obviously increased and arranged disorderly. In some smooth muscle cells, rough endoplasmic reticulum, ribosomes and Golgi bodies were increased while myofilaments reduced, indicating that phenotypes of smooth muscle cells were changed from constrictive to synthetic type (Figures 2A and 2B). Intima of the normal splenic vein was smooth and endothelial cells were not damaged. In the patients with hepatic cirrhosis, intima of the splenic vein wall was damaged and vascular smooth muscle cells of synthetic type were predominant. Cytoplasma of the smooth muscle cells had plentiful rough endoplasmic reticulum and Golgi bodies. Along the vascular wall, a lot of collagenous fibers were deposited and disorderly arranged (Figures 2C and 2D). Under scanning electron microscope, intima was smooth and endothelial cells were intact in the normal splenic vein (Figure 3A). In the patients with hepatic cirrhosis, intima of the splenic vein was damaged, endothelial cells were not intact, blood components accumulated, surface of the endothelia was irregular(Figures 3B and 3C). In the normal splenic artery, intima was intact and endothelial cells were not destroyed (Figure 4A). In the patients with hepatic cirrhosis, intima of the splenic artery was damaged and red blood cells and platelets accumulated around the damaged area (Figures 4B and 4C).
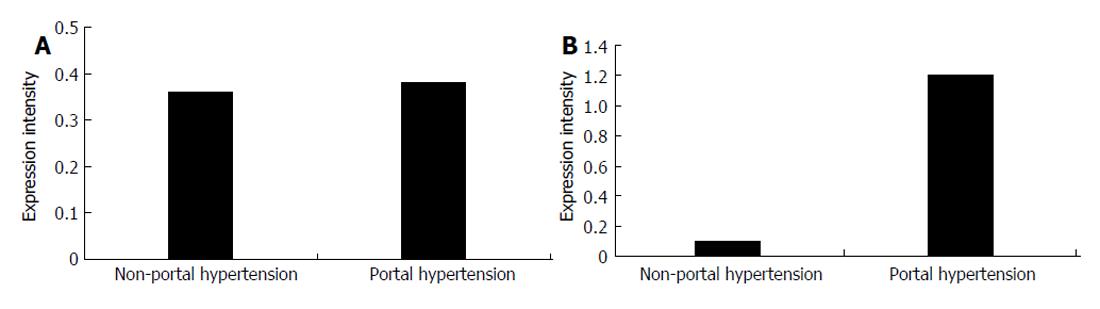
In splenic vein wall of the patients with portal hypertension, the expression intensity of type I procollagen mRNA was 0.3868 ± 0.0358, while that in the patients without hepatic cirrhosis was 0.3642 ± 0.0332 with no significant difference (P > 0.05). In portal vascular wall of the patients with portal hypertension, the expression intensity of type III procollagen mRNA was 1.1855 ± 0.2140, while that in the patients without hepatic cirrhosis was 0.1095 ± 0.1558 with significant difference (P < 0.01, Figure 5 and Figure 6)
Serious damage to the intima in patient with portal hypertension results from increased portal pressure and blood flow. It has been reported that phenotype of vascular smooth muscle cells changes from normal constrictive to synthetic type[3,4] due to increased cytokines[5,6], growth factor[7], shearing force[8,9], oxygen stress[10]. The expression of constrictive proteins in vascular smooth muscle cells is inhibited, and a plentiful of extra-cellular matrix (ECM) is produced and migrates into subintima. In this study, under optical microscope, the tunica media was thickened, which is an adaptative compensatoty response of vessels to the increased pressure. Smooth muscle cells in tunica media of the splenic artery were degenerated and became shrunk and their phenotype changed from constrictive to synthetic type, which is attributed to the ischemic vascular wall injury by insufficient blood supply due to compression of nutrient vessels resulting from the increased pressure and flow. Under scanning electron microscope, damage to intima of the splenic artery was seen, which is associated with the portal system involvement. In addition, under optical microscope, internal elastic membrane of the splenic artery was broken due to the following causes, such as splanchnic hyperdynamic circulation, increased blood flow and velocity, increased wall pressure and shear stress on vascular wall, long-term vascular congestion and dilation, thus resulting in endothelial exfoliation and rupture of elastic membrane. The broken internal elastic membrane contributes to the decreased ability of vascular wall to resist the blood flow attack. When the blood flow is increased, the vessels are not constricted and the blood flow is not regulated by elastic membrane, which is beneficial to the development of hyperdynamic circulation.
Collagen is an important vascular extra-cellular matrix and can maintain the flexibility and intensity of vascular wall. In this study, expression of type III procollagen mRNA in the splenic vein wall was about 5-6 times higher in patients with portal hypertension than in those without portal hypertension (P < 0.01), while there was no significant difference in the expression of type I procollagen mRNA between the patients with and with portal hypertension (P > 0.05). We believe that the high expression of type III porcollagen mRNA in patients with portal hypertension might be an important factor promoting vascular remodeling. At present, it is unclear about the modulation mechanism of type I and III procollagen gene expression. It has been reported that many factors, such as FGF[11], Ang-II[12] can up-regulate type I and III procollagen mRNA in vascular smooth muscle cells.
In conclusion, type III porcellagen and collagen may be an important extracellular matrix resulting in vascular remodeling in patients with portal hypertension. Splenic vasculopathy exists in patients with portal hypertension, while intrahepatic blood flow obstruction resulting from hepatic cirrhosis is the initial factor responsible for the development of portal hypertension. On the one hand, portal hypertension can promote the formation and development of splanchnic hyperdynamic circulation and splanchnic vasculapathy. On the other hand, splanchnic vasculapathy can enhance the formation and development of portal hypertension, as well as promote splanchnic hyperdynamic circulation. These three factors play an important role in the pathogenesis of cirrhotic portal hypertension and are involved in variceal hemorrhage.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Yang Z, Ren D, Li D, Qiu F. [Portal hypertensive vasculopathy of splenic artery]. Zhonghua Waike Zazhi. 1999;37:412-414. [PubMed] |
| 2. | Yang Z, Zhang L, Li D, Qiu F. Pathological morphology alteration of the splanchnic vascular wall in portal hypertensive patients. Chin Med J (Engl). 2002;115:559-562. [PubMed] |
| 3. | Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421-1430. [PubMed] |
| 4. | Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Jiang B, Xu S, Brecher P, Cohen RA. Growth factors enhance interleukin-1 beta-induced persistent activation of nuclear factor-kappa B in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1811-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | King KE, Iyemere VP, Weissberg PL, Shanahan CM. Krüppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem. 2003;278:11661-11669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Wu J, Cunnick JM. Trans-regulation of epidermal growth factor receptor by lysophosphatidic acid and G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Hosokawa H, Aiuchi S, Kambe T, Hagiwara Y, Kubo T. Mechanical stretch-induced mitogen-activated protein kinase activation is mediated via angiotensin and endothelin systems in vascular smooth muscle cells. Biol Pharm Bull. 2002;25:1588-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Standley PR, Cammarata A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol. 2002;283:H1907-H1914. [PubMed] |
| 10. | Komai N, Morishita R, Yamada S, Oishi M, Iguchi S, Aoki M, Sasaki M, Sakurabayashi I, Higaki J, Ogihara T. Mitogenic activity of oxidized lipoprotein (a) on human vascular smooth muscle cells. Hypertension. 2002;40:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Pickering JG, Ford CM, Tang B, Chow LH. Coordinated effects of fibroblast growth factor-2 on expression of fibrillar collagens, matrix metalloproteinases, and tissue inhibitors of matrix metalloproteinases by human vascular smooth muscle cells. Evidence for repressed collagen production and activated degradative capacity. Arterioscler Thromb Vasc Biol. 1997;17:475-482. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Touyz RM, He G, El Mabrouk M, Schiffrin EL. p38 Map kinase regulates vascular smooth muscle cell collagen synthesis by angiotensin II in SHR but not in WKY. Hypertension. 2001;37:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |