Published online Apr 28, 2006. doi: 10.3748/wjg.v12.i16.2615
Revised: January 2, 2006
Accepted: January 9, 2006
Published online: April 28, 2006
AIM: To elucidate the role of Wnt/β-catenin signaling pathway in pancreatic development of rat embryo.
METHODS: The mRNAs of β-catenin, APC, cyclin D1 genes were amplified by means of semiquantitative reverse transcription polymerase chain reaction (RT-PCR) from embryonic pancreas in different periods and normal pancreas of rat, respectively. Protein expression of these genes in embryonic pancreas of E14.5-E18.5 was examined by immunohistochemical method.
RESULTS: In embryonic pancreas of E14.5, the transcript amplification of β-catenin and cyclinD1 genes was detected. In embryonic pancreas of E18.5, the transcription levels of β-catenin and cyclinD1 genes became much higher than in other periods. But in adult rat pancreas the transcription of cyclinD1 gene could not be observed. Only until E18.5, the transcript amplification of mRNA of APC gene could be detected. Surprisingly, the transcription level of APC gene became much higher in adult rat pancreas than in embryonic pancreas. By means of immunohistochemical staining, identical results were obtained to the above by RP-PCR, except for β-catenin protein in adult rat pancreas.
CONCLUSION: Active Wnt/β-catenin signaling occurs in rat embryonic pancreas and is probably important for pancreatic development and organ formation.
- Citation: Wang QM, Zhang Y, Yang KM, Zhou HY, Yang HJ. Wnt/β-catenin signaling pathway is active in pancreatic development of rat embryo. World J Gastroenterol 2006; 12(16): 2615-2619
- URL: https://www.wjgnet.com/1007-9327/full/v12/i16/2615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i16.2615
The fetal pancreatic development begins at the end of fourth week of gestational period in human being. The pancreatic bud comes from entodermal epithelium in the foregut. In rat embryo the pancreatic bud occurs at 9.5 d after mating (E9.5)[1]. With pancreatic islet arising, the rat embryonic pancreas began to become a special organ including external secretory portion and internal secretory portion. E14.5 - E18.5 is a crucial period for rat pancreatic cell proliferation, differentiation and structure formation[2-3]. Wnt/β-catenin signaling is involved in many developmental processes such as proliferation, differentiation, cell fate decisions, and morphogenesis[4]. However, little is known about Wnt/β-catenin signaling during pancreas development. β-catenin serves not only as a structural component of the E-cadherin-mediated cell-cell adhesion system, but also a signaling molecule of the Wnt/β-catenin pathway. In present study, we tried to investigate the role of Wnt/β-catenin signaling pathway in rat pancreatic development by means of RT-PCR and immunohistochemical method for collecting more data on diagnosis and therapy of pancreatic diseases.
Healthy SD rats (male 10 and female 20), weighing 200-250 g, were purchased from the experimental animal center in Huaxi University of Medical Sciences, Chendu, China. Every two female rats and one male rat was put into one cage after these animals were fed for a week. Assessment of the embryonic age of the fetuses was based on the plug date, defined as embryonic d 0 (E0). The embryos were harvested from female rats with E14.5, E15.5, E16.5, E17.5 and E18.5, respectively. A part of pancreases was rapidly frozen in liquid nitrogen as soon as they were dissected from embryos under microscope.
Kits used in this study were as follows: Total RNA extraction kit (W6701, Watson, Shanghai), reverse transcription kit (RevertAid HMinus First Strand cDNA Synthesis Kit, Fermentas), and PCR reaction kit (DRR01AM, TaKaRa, Japan).
The following antibodies were used in this study: Rabbit polyclonal antibody against ββ-catenin, mouse monoclonal antibody against cyclin D1, rabbit polyclonal antibody against APC (Santa Cruz, USA).
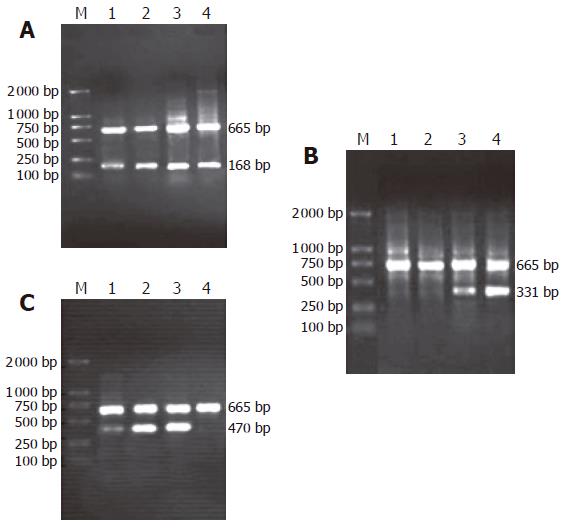
Semiquantitative PCR was performed to determine the levels of the mRNA transcripts encoding β-catenin, APC and cyclinD1 genes in embryonic pancreas on E14.5-E18.5. The published sequences of the primers for amplification of β-catenin, APC, cyclinD1 and the housekeeping gene β-actin are showed in Table 1. To determine the optimum number of cycles required for the amplification of these genes or β-actin, an aliquot of first strand cDNA generated from normal rat pancreas was amplified with the respective primers using an increasing number of PCR cycles (20-36). To avoid primer-dependent artifacts, the reaction mixtures were denatured at 95 °C for 5 min prior to the addition of the Taq polymerase. The subsequent cycling programs consisted of denaturation at 95 °C for 30s, annealing at 60 °C (Table 1) (β-actin for 30 seconds) and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 8 min. A linear relationship between the band intensity of the PCR products and the number of amplification cycles performed was observed. Based upon these observations, the optimum numbers of cycles for the amplification of these genes were 28-30 cycles and of β-actin was 28 cycles. PCR reactions in which the first strand cDNA were omitted served as negative controls and cDNA generated from endometrial samples were used as positive controls for these studies. To avoid technical error, each PCR experiment has been repeated twice. The PCR products were separated on 1.5% agarose gels, stained with ethidium bromide, and photographed using Gel Imaging System (BioRad, USA). The intensity of the bands specific for each target gene or β-actin was quantified using the Phoretix Gel Analysis Software Version 3.01 (NonLinear Dynamics, UK). The relative mRNA levels of these genes for each sample obtained from embryonic pancreas of E14.5-E18.5 were normalized to the corresponding β-actin levels.
| Genes | Sequence of primer (5’→3’) | Length ofproduction(bp) | Annealingtemperature(Tm) |
| F: ACAGCACCTTCAGCACTCT | |||
| β-catenin | R: AAGTTCTTGGCTATTACGACA | 168 | 58.2 |
| F: CGGAACATGCATGACTGAGAC | |||
| APC | R: GTCACGAGGTACGACCTCAGAT | 310 | 60 |
| F: CAGAAGTGCGAAGCTTAGGTCT | |||
| cyclin D1 | R: GTAGCAGGAGAAGTTGTTGG | 470 | 58 |
| F: CATGTGCAAGGCCGGCTTCG | |||
| β-actin | R: GTAGCAGGAGAAGTTGTTGG | 665 | 60 |
The avidin-biotin peroxidase complex (ABC) technique was used for immunohistochemical staining. Sections were cut at 5µm thickness, deparaffinized in xylene, rehydrated and washed with water. They were treated with 3% hydrogen peroxidase for 20 min to quench endogenous peroxidase and heated in a citrate buffer solution (0.1 mol/L sodium citrate, pH 6.0) at 95°C for 10 min. After pre-incubation with 10% normal goat serum to block non-specific binding, sections were incubated with the primary antibodies against β-catenin, APC and cyclin D1 at 4°Covernight. Alternatively, the sections were incubated with biotinylated anti-rabbit or mouse IgG (dilution of 1∶200) for 40 min at room temperature and with ABC (dilution of 1∶200) for 30 min at room temperature. Between incubations, sections were washed with 0.1 mol/L PBS (pH7.4). Color was developed with diaminobenzidine tetrahydrochloride supplemented with 0.04% hydrogen peroxidase and counterstained with Mayer’s hematoxylin.
The results of semiquantitative RT-PCR are presented as the ratio of the mean relative absorbance of β-catenin, APC and cyclin D1 to corresponding β-actin respectively for at least three independent experiments of each sample. Statistical differences among different periods of embryo were assessed by t test. A P < 0.05 was considered significant.
As shown in Figure 1(A-C), the amplification bands of mRNA of β-catenin and cyclinD1 genes could still be detected in rat embryonic pancreas from E14.5-E18.5. In adult rat pancreas the transcription of cyclinD1 gene could not be observed while the transcription of β-catenin gene could be found. Only in pancreas of E18.5, the transcript amplification of mRNA of APC gene could be detected. Surprisingly, the transcription level of APC gene was higher in adult rat pancreas than in embryonic pancreas.
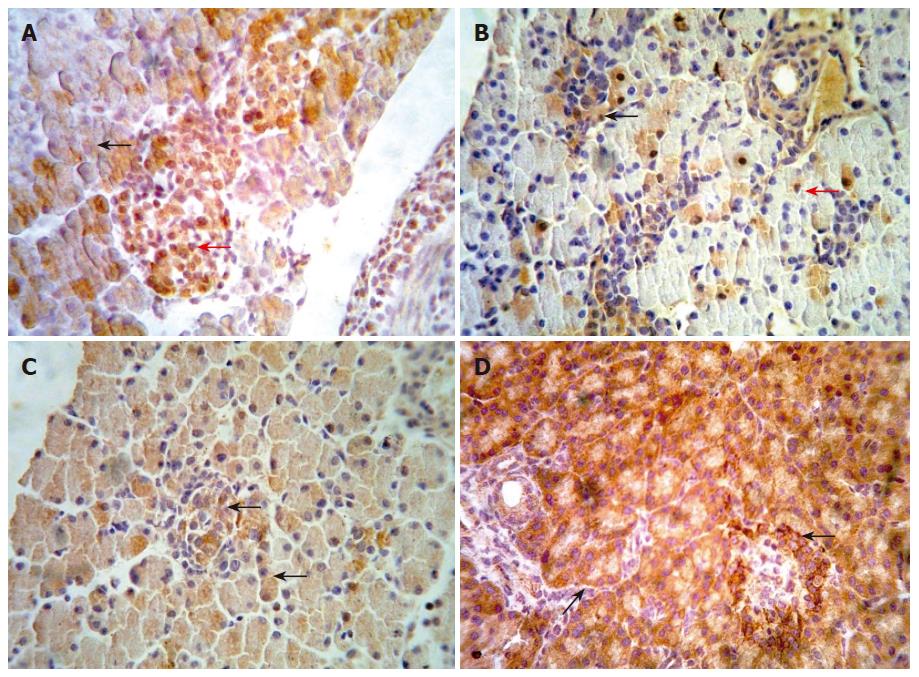
We examined the β-catenin, cyclinD1 and APC protein in embryo from E14.5-E18.5 by immunohistochemical staining. Representative results are shown in Figure 2 (A-D). In the embryo of E14.5, β-catenin staining appeared at the cytoplasm of pancreatic cells. The cells with positive staining showed a diffuse distribution in embryonic pancreas. In the embryo of E18.5, we could observe full pancreas including external and internal secretion portions in morphology. At the same time, our results showed that β-catenin could be detected frequently both in external and internal secretion portions. The positive cells of cyclinD1 with diffuse distribution were observed in the pancreas of E14.5. The positive staining located in the cytoplasm and/or nuclei of pancreatic cells. In pancreas of E18.5, the expression of cyclinD1 protein was significantly higher than that in other period. However, none of positive cells expressing cyclinD1 protein was observed in adult pancreas. Expression of APC has not been observed in the early embryonic pancreas until E18.5. In contrast, the positive cells of APC staining scattered throughout the pancreas in adult rat.
At present, morbidity rate of diabetes has an ascending tendency in the world while high morbidity and mortality of pancreatic carcinoma is becoming one formidable topic for surgeon[5]. In order to treat these diseases effectively, fully understanding of the development and differentiation of embryonic pancreas, as well as the underlying molecular mechanism of pancreatic diseases is needed.
Wnt/β-catenin signaling pathway is highly conservative during embryonic development and tumorigenesis of human being and animal[6]. Recent studies have suggested that activation of Wnt/β-catenin signaling pathway may play an important role in hematonosis[7], gastroenteric tumor such as colon cancer[8], and development of embryo[9]. In this study, we have investigated the transcription of gene and expression of protein of β-catenin, APC and cyclin D1 which are three important components in Wnt/β-catenin signaling pathway.
β-catenin serves not only as a structural component of the E-cadherin-mediated cell-cell adhesion system, but also as a signaling molecule of the Wnt/β-catenin signaling pathway. The β-catenin protein is at the core of the canonical Wnt/β-catenin signaling pathway. Wnt stimulation leads to β-catenin accumulation, nuclear translocation and interaction with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to regulate genes important for embryonic development and proliferation. In a word, accumulation of β-catenin protein in the cytoplasm and/or nuclei of cell are popularly considered a hallmark of activation of the canonical Wnt/β-catenin signaling pathway[7,10]. At this study, we have observed the transcription of β-catenin gene by the RT-PCR and positive stain of β-catenin protein with immunohistochemical techniques in pancreas of E14.5-E18.5. Moreover the positive cells of β-catenin existed in not only the external secretion portion but also internal secretion portion. Therefore we presumed that Wnt/β-catenin signaling pathway may be very important for morphogenesis of pancreas. In pancreas of E18.5, the positive stain of β-catenin protein could be found in nucleus especially in pancreatic islet. We have known that pancreatic islet began to mature during late period of embryo[11]. So β-catenin may be essential for proliferation and differentiation of pancreas islet in embryo. Dessimoz et al[12] examined the role of β-catenin gene in development of pancreas using an animal model with the conditional gene knockout.They found a reduction in endocrine islet numbers after deleting the β-catenin gene in the epithelium of the pancreas. This result in some degree coincided with our conclusion. On contrast, Murtaugh et al[13] confirmed β-catenin is essential only for pancreatic acinar not for islet development. Obviously the development process of pancreas is too complicated to be understood only by Wnt/β-catenin signaling.
In the study, we investigated simultaneously cyclin D1 gene which is one of target genes of Wnt/catenin signaling pathway[14]. The changed tendency of cyclin D1 on the transcription level of mRNA and protein expression is almost in step with β-catenin in pancreas of E14.5-E18.5. It is known that cyclin D1 is a key regulator of the G1 phase of cell cycle[15]. Expressing of cyclin D1 protein in the cytoplasm and/or nuclei indicated that pancreatic cells should be in the condition of proliferation and differentiation. We therefore had come to another conclusion that cyclin D1 exactly is one of activated target genes of Wnt signaling pathway in development of embryonic pancreas.
It has been reported that APC is a negative regulatory factor as well as Axin in Wnt signaling pathway[16]. Experiments in Drosophila ultimately revealed that genetic ablation of APC indeed resulted in upregulation of β-catenin signaling[17]. In pancreas of E14.5-17.5, we could not observe the amplification band of mRNA and protein expressing of APC gene. Only in pancreas of E18.5, could we first find positive stain of APC protein as well as transcription of mRNA of APC gene. In adult rat the positive cells of APC protein spread throughout external secretion portion and internal secretion portion of pancreas. We have known that the proliferation level of pancreas is very low in adult rat particularly in diabetes[18]. This is coincident with the negative role of APC in Wnt/β-catenin signaling pathway.
In this study the cell with positive stain of β-catenin couldn’t be found in adult rat pancreas although the transcription of β-catenin gene could be detected by RT-PCR. The reasons are discussed below. First the destruction complex including GSK3、Axin and APC can degradate the free β-catenin in the cytoplasm in time in adult rat pancreas[19]. Secondly the quantity of β-catenin protein which are attached to membrane with E-cadherin may be too little to be detected by immunohistochemical techniques used in present experiments[20].
Although this signaling pathway was active, we had known little about the role of Wnt /β-catenin signaling pathway in development of embryonic pancreas indeed. Which are the upperstream signals[21-22] Which target genes beside of cyclin D1 are activated How to crosstalk with other signaling pathway[23] All these topics need us to investigate further.
Meanwhile, some workers have investigated the Wnt/β-catenin signaling in tumor of pancreas. They suggested the notion that Wnt/β-catenin signaling pathway had been activated in adenocarcinoma, cystocarcinoma and solid tumor of pancreas[24-26]. We will believe that the more we know the Wnt/β-catenin signaling in embryonic pancreas and tumor of pancreas, the better the therapeutic measures for patient with diabetes and tumor of pancreas clinically.
S- Editor Wang J L- Editor Zhang JZ E- Editor Liu WF
| 1. | Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | St-Onge L, Wehr R, Gruss P. Pancreas development and diabetes. Curr Opin Genet Dev. 1999;9:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. [PubMed] |
| 4. | Pedersen AH, Heller RS. A possible role for the canonical Wnt pathway in endocrine cell development in chicks. Biochem Biophys Res Commun. 2005;333:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Serup P, Madsen OD, Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ. 2001;322:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Behrens J. Control of beta-catenin signaling in tumor development. Ann N Y Acad Sci. 2000;910:21-33; discussion 33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 180] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Kolligs FT, Bommer G, Göke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Yamaoka T, Itakura M. Development of pancreatic islets (review). Int J Mol Med. 1999;3:247-261. [PubMed] |
| 12. | Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of beta-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr Biol. 2005;15:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development. 2005;132:4663-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun. 2005;330:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Farr GH 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Holland AM, Hale MA, Kagami H, Hammer RE, MacDonald RJ. Experimental control of pancreatic development and maintenance. Proc Natl Acad Sci U S A. 2002;99:12236-12241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | von Kries JP, Winbeck G, Asbrand C, Schwarz-Romond T, Sochnikova N, Dell'Oro A, Behrens J, Birchmeier W. Hot spots in beta-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol. 2000;7:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Tien LT, Ito M, Nakao M, Niino D, Serik M, Nakashima M, Wen CY, Yatsuhashi H, Ishibashi H. Expression of beta-catenin in hepatocellular carcinoma. World J Gastroenterol. 2005;11:2398-2401. [PubMed] |
| 21. | Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, Serup P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 792] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Skromne I, Stern CD. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915-2927. [PubMed] |
| 24. | Miao J, Kusafuka T, Kuroda S, Yoneda A, Zhou Z, Okada A. Mutation of beta-catenin and its protein accumulation in solid and cystic tumor of the pancreas associated with metastasis. Int J Mol Med. 2003;11:461-464. [PubMed] |
| 25. | Pujal J, Capellá G, Real FX. The Wnt pathway is active in a small subset of pancreas cancer cell lines. Biochim Biophys Acta. 2006;1762:73-79. [PubMed] |
| 26. | Lowy AM, Fenoglio-Preiser C, Kim OJ, Kordich J, Gomez A, Knight J, James L, Groden J. Dysregulation of beta-catenin expression correlates with tumor differentiation in pancreatic duct adenocarcinoma. Ann Surg Oncol. 2003;10:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |