Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2406
Revised: November 2, 2005
Accepted: November 18, 2005
Published online: April 21, 2006
AIM: To assess whether portacaval anastomosis (PCA) in rats affects the protein expression and/or activity of glutaminase in kidneys, intestines and in three brain areas of cortex, basal ganglia and cerebellum and to explain the neurological alterations found in hepatic encephalopathy (HE).
METHODS: Sixteen male Wistar rats weighing 250-350 g were grouped into sham-operation control (n = 8) or portacaval shunt (n = 8). Twenty-eight days after the procedure, the animals were sacrificed. The duodenum, kidney and brain were removed, homogenised and mitochondria were isolated. Ammonia was measured in brain and blood. Phosphate-activated glutaminase (PAG) activity was determined by measuring ammonia production following incubation for one hour at 37 °C with O-phthalaldehyde (OPA) and specific activity expressed in units per gram of protein (µkat/g of protein). Protein expression was measured by immunoblotting.
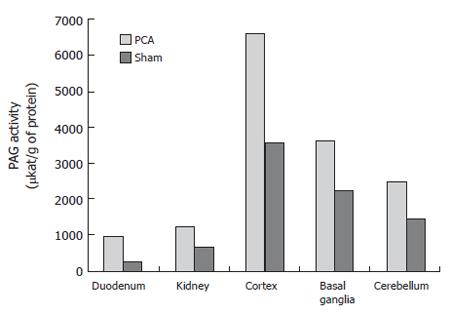
RESULTS: Duodenal and kidney PAG activities together with protein content were significantly higher in PCA group than in control or sham-operated rats (duodenum PAG activity was 976.95±268.87 µkat/g of protein in PCA rats vs 429.19±126.92 µkat/g of protein in sham-operated rats; kidneys PAG activity was 1259.18 ± 228.79 µkat/g protein in PCA rats vs 669.67± 400.8 µkat/g of protein in controls, P < 0.05; duodenal protein content: 173% in PCA vs sham-operated rats; in kidneys the content of protein was 152% in PCA vs sham-operated rats). PAG activity and protein expression in PCA rats were higher in cortex and basal ganglia than those in sham-operated rats (cortex: 6646.6 ± 1870.4 µkat/g of protein vs 3573.8 ± 2037.4 µkat/g of protein in control rats, P < 0.01; basal ganglia, PAG activity was 3657.3 ± 1469.6 μkat/g of protein in PCA rats vs 2271.2 ± 384 μkat/g of protein in sham operated rats, P < 0.05; In the cerebellum, the PAG activity was 2471.6 ± 701.4 μkat/g of protein vs 1452.9 ± 567.8 μkat/g of protein in the PCA and sham rats, respectively, P < 0.05; content of protein: cerebral cortex: 162% ± 40% vs 100% ± 26%, P < 0.009; and basal ganglia: 140% ± 39% vs 100% ± 14%, P < 0.05; but not in cerebellum: 100% ± 25% vs 100% ± 16%, P = ns).
CONCLUSION: Increased PAG activity in kidney and duodenum could contribute significantly to the hyperammonaemia in PCA rats, animal model of encephalopathy. PAG is increased in non-synaptic mitochondria from the cortex and basal ganglia and could be implicated in the pathogenesis of hepatic encephalopathy. Therefore, PAG could be a possible target for the treatment of HE or liver dysfunction.
- Citation: Romero-Gómez M, Jover M, Díaz-Gómez D, Terán LC, Rodrigo R, Camacho I, Echevarría M, Felipo V, Bautista JD. Phosphate-activated glutaminase activity is enhanced in brain, intestine and kidneys of rats following portacaval anastomosis. World J Gastroenterol 2006; 12(15): 2406-2411
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2406
Hyperammonaemia plays a major role in the pathogenesis of hepatic encephalopathy (HE). Phosphate-activated glutaminase (PAG) catalyzes the hydrolysis of glutamine (Gln) to yield glutamate (Glu), energy, nucleotide synthesis and ammonia. Two main isoforms of PAG have been described: the kidney-type (K-PAG) and the liver type (L-PAG). The K-PAG has been found in kidney, brain and villi enterocytes, while L-PAG is restricted to the liver. Hyperammonaemia is largely considered to be derived from urea breakdown by intestinal bacteria, thus most treatments have been focussed on reducing ammonia production from colon bacteria[1]. However, some data suggest that small intestine plays an important role in ammonia production during the pathogenesis of hepatic encephalopathy[2]. Indeed, duodenal PAG activity has been found to be nearly four times higher in cirrhotic patients than in healthy controls, and moreover it is closely related to minimal hepatic encephalopathy[3]. In rats, the highest PAG activity along the length of gastrointestinal tract has been measured in the small intestine[4]. Lower but still substantial activity (15% of total PAG activity) has been found in large intestine. PAG activity distribution along the gastrointestinal tract in humans is similar to that in rats[5]. PAG plays another important role at renal level. Glutamine is filtered and reabsorbed in the proximal convoluted tubule where it is deamidated by PAG. Two thirds of this ammonia production is excreted in urine. This process is pH sensitive and helps to maintain acid-base homeostasis and to excrete nitrogen[6]. However, PAG activity in kidney remains largely unexplored in portacaval shunted rats. Distribution and location of PAG in brain as well as its role in the pathogenesis of HE, are widely controversial, while PAG activity in portacaval shunted rats still remains unknown. Portacaval anastomosis (PCA; or portacaval shunt PCS) in rats is widely accepted as a model of type B hepatic encephalopathy that mimics minimal hepatic encephalopathy in humans. The aim of this study was to assess whether portacaval anastomosis in rats affects the expression and/or activity of glutaminase in kidney, intestines and the astrocytes of three brain areas (cortex, basal ganglia and cerebellum) involved in the neurological alterations of hepatic encephalopathy.
Sixteen male Wistar rats (n = 16) weighing 250-350 g were randomly allocated into sham operation controls (n = 8) or portacaval shunt (n = 8). All animals were pair fed two weeks before and four weeks after operation and kept under standard laboratory conditions. Both PCA and sham-operated rats were fasted overnight in single wire-net floor cages with free access to tap water. All animal procedures were approved by our institution and met the guidelines of Spain (RD 223 erased 14th March 1998) and European (Directive 86/609/CEE) Union for care and management of experimental animals.
Rats were operated under general anaesthesia with isofluorane to avoid liver metabolism of intravenous agents and end-to-side portacaval shunt was performed as previously described[7]. Briefly, after middle laparotomy for viscera exteriorization, inferior vena cava and portal veins were exposed, dissected and clamped laterally together with a Satinsky clamp. Longitudinal incisions in both veins and lateral anastomosis with running suture as usual were performed. Finally, portal trunk was tied and cut next of liver hilum, turning the portacaval lateral shunt in functionally terminal. Sham-operation was performed following laparotomy, the inferior vena cava was isolated and clamped for 30 s. Sham-operated animals served as controls.
Ammonia was measured in cerebral cortex and blood as previously described[8]. Cerebral cortex was homogenized and deproteinised in 5 volumes of ice-cold 100 g/L trichloroacetic acid, and kept on ice for 15 min. After centrifugation at 12 000 r/min for 10 min at 4 °C, the supernatants were collected, neutralized with 2 mol/L KHCO3 and centrifuged at 12 000 r/min for 10 min at 4 °C. The neutralized supernatants were used to measure ammonia in µmole/g tissue. Blood (150 µL) was taken from the tail vein the third week after surgery. Blood samples were deproteinized with one volume of ice-cold 100 g/L trichloroacetic acid and kept on ice for 15 min. After centrifugation at 12 000 r/min for 10 min at 4 °C, the supernatants were collected, neutralized with 2 mol/L KHCO3 and centrifuged at 12 000r/min for 10 min at 4 °C. The neutralized supernatants were used to measure ammonia. In a final volume of 100 µL, the reaction mixture contained 50 µL or 60 µL of sample, 30 mmol/L α-ketoglutarate, 0.5 mmol/L nicotinamide adenine dinucleotide (reduced form) in potassium phosphate buffer (pH 8.0). After recording of the initial fluorescence, reactions were started by the addition of 5 µg of glutamate dehydrogenase (Boehringer Mannheim, Germany) and monitored by the fluorimeter (Fluoroskan Ascent; Labsystems; Oy, Helsinki, Finland) for at least 70 min. Standards containing up to 25 nmol of ammonia were included in each assay. Assays were performed in Costar 96-well UV plates (cat. No. 3635; Corning Costar Corporation, Cambridge, MA).
All procedures were carried out in the cold room at 2°C - 4°C. The rats were killed by cervical dislocation. The skull of each rat was opened to remove blood from the surface of the tissue and this procedure needed to be performed in <30 s. The tissue was placed in 5 mL of isolation medium in a Petri dish maintained in an ice bath. The brain was chopped with fine scissors and the chopped material was washed frequently with isolation medium to remove blood. Meanwhile, the first portion of small intestine (3 cm of length) was removed and placed in 5 mL of isolation medium in Petri dish maintained in an ice bath. The duodenum was washed and mucosa was removed by glass film and quickly frozen in liquid air. Samples of kidney were similarly obtained. Tissue samples were then homogenized manually in 1 mL of isolation medium per 100-150 mg of tissue using a Dounce homogenizer fitted with a Teflon pestle having a total clearance of 0.1 mm. Usually, six up- and down-strokes were sufficient to generate a rough homogenate which was then diluted with isolation medium to a final volume of 1.2 mL and homogenized further with four up- and down-strokes.
The procedure for isolation of brain mitochondria was based on previously reported methods[9] except for 1 mmol/L ethylene glycol-bis (β-aminoethyl ether) tetra-acetic acid (EGTA) being used in the homogenisation medium instead of EDTA. The homogenate was centrifuged at 2 000 r/min for 3 min, the pellet was washed with 400 μL of homogenisation medium and re-centrifuged at 2 000 r/min for 3 min. Both supernatants were pooled and centrifuged for 8 min at 12 000 r/min to obtain the crude mitochondrial pellet. The pellet was suspended in 300 μL of the 30 g/L Ficoll medium (see below) and layered onto 1.2 mL of 60 g/L Ficoll medium and centrifuged at 12 000 r/min for 30 min. The 60 g/L Ficoll medium contained 6% (w/w) Ficoll, 0.24 mol/L mannitol, 0.06 mol/L sucrose, 0.05 mmol/L K-EDTA and 10 mmol/L Tris-HCl, pH 7.4. The 30 g/L Ficoll medium was the 60 g/L Ficoll medium diluted 1 : 1 with glass re-distilled water. The loose, fluffy, white upper layer of the pellet was removed, the remaining brown pellet was re-suspended in isolation medium without EGTA and the suspension was centrifuged at 12 000 r/min for 10 min. The pellet was re-suspended in incubation medium (isolation medium with 2.5 mL/L buffer containing protease inhibitor, 7 g/L Triton X-100, 5 mmol/L β-mercapto-ethanol) to obtain a protein concentration in the range of 5-10 g/L. After incubation on a mixing wheel for 30 min at 4 °C, the samples were frozen at -80 °C for batched activity measurement.
Mitochondrial protein was measured by the method of Bradford et al[10] with bovine serum albumin (BSA) as standard. Briefly, 25 μL of mitochondrial solution was added to 35 μL of reaction medium (150 mmol/L K2HPO4, pH 8; 171 mmol/L L- GLn; 1 mmol/L NH4Cl; pH 8). After incubation for 60 min the reaction was stopped with 10 μL of 100 g/L trichloroacetic acid (TCA). Blanks were prepared separately following the incubation of the reaction medium and samples were mixed before the addition of TCA. When the sample-mixture reaction was stopped, the reaction mixture was placed in ice for 15 min and then centrifuged at 12 000 r/min for 5 min at 4 °C. The micro-titre plate was loaded with 5 μL of supernatant and 150 μL of OPA reagent (0.2 mol/L K2HPO4, pH 7.4; 56 mL/L ethanol; 10 mmol/L O-phthaldialdehyde; 0.4 mmol/L β-mercapto-ethanol). The plate was incubated in dark at room temperature for 45 min. Standards of NH4Cl were prepared to concentrations of 50-300 mg/L. Absorbance was measured at 405 nm with a spectrophotometer (R&D System, Palo Alto, USA). Specific activities of enzymes were expressed in international units per gram (μkat/mg) of mitochondrial homogenate protein.
Samples from sham-operated rats or rats with PCS were homogenized in medium containing 66 mmol/L Tris-HCl (pH 7.4), 10 g/L SDS, 1 mmol/L EGTA, 100 ml/L glycerol, 1 mmol/L sodium orthovanadate and 1 mmol/L sodium fluoride and the protein concentration was determined by the bicinchonic acid method (Pierce, Rockford, IL, USA). Samples were subjected to gel electrophoresis and immunoblotting as previously described[11] using isoform-specific polyclonal antibodies raised in rabbits against K- glutaminase proteins diluted at 1 : 1000. After incubation with anti-rabbit IgG conjugated with alkaline phosphatase (Sigma, Germany) and development with alkaline phosphatase colour developer (Sigma, Germany), the image was captured using the Gel Printer Plus System (TDI, Madrid, Spain) and the densities of the spots were measured using the Intelligent Quantifier™ software Version 2.5.0 (BioImage®, Madrid, Spain). Results were relativized to the optical density respect to controls.
Data were expressed as mean ± SD. Statistical analyses were performed using the SPSS 11.0 software (spss, Chicago, IL). Differences in glutaminase activity or protein content were analysed by Student t-test. P ≤ 0.05 was considered statistically significant for all tests applied.
Ammonia was significantly higher in PCA group than in control rats. Plasma ammonia level was 166 ± 51 µmol/L in PCA rats and 83 ± 12 µmol/L in control rats (P < 0.05). In the cortex, brain ammonia was 0.9±0.4 µmol/g of tissue in PCA rats and 0.3 ± 0.1 µmol/g of tissue in sham operated rats (P < 0.05).
Duodenal and kidney PAG activities were significantly higher in PCA group than in control rats. In the duodenum, PAG activity was 976.95 ± 268.87 μkat/g of protein in PCA rats and 429.19 ± 126.92 μkat/g of protein in sham-operated rats (P < 0.05). In mitochondria from kidneys, PAG activity was 1259.18 ± 228.79 μkat/g protein in PCA rats and 669.67 ± 400.8 μkat/g of protein in controls (P < 0.05).
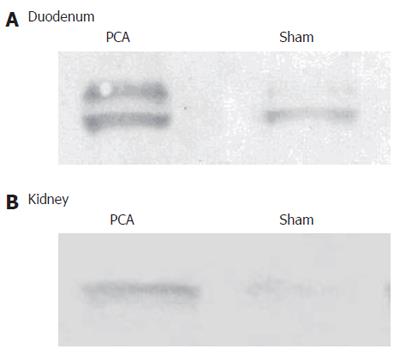
There was a significant effect of PCA on PAG protein in duodenum. The content of protein was 173% in PCA compared to sham-operated rats and in kidneys the content of protein was 152% in PCA compared to sham-operated rats (Figure 1).
The PAG activity was 6646.6 ± 1870.4 μkat/g of protein in the cortex of PCA rats and 3573.8 ± 2037.4 μkat/g of protein in that of control rats (P < 0.01). The PAG activity was 3657.3 ± 1469.6 μkat/g of protein in basal ganglia of PCA rats and 2271.2 ± 384 μkat/g of protein in that of sham-operated rats (P < 0.05). In the cerebellum, the PAG activity was 2471.6 ± 701.4 μkat/g of protein and 1452.9 ± 567.8 μkat/g of protein in the PCA and sham-operated rats, respectively (P < 0.05). In PCA rats, the PAG activity was increased up to 186% in cortex, 161% in basal ganglia and 170% in cerebellum compared to sham-operated rats. The highest activity was found in the cortex (Figure 2).
The content of glutaminase was significantly increased in cerebral cortex of PCA rats compared to sham-operated animals (162% ± 40% and 100% ± 26%; P < 0.009) and the content of glutaminase was significantly higher in basal ganglia of PCA rats than in that of sham-operated animals (140% ± 39% and 100%±14%; P < 0.024), but there was no significant difference between the groups after PCA in cerebellum (100% ± 25% and 100% ± 16%; P = NS) (Figure 3).
The PCA performed in rats is widely accepted as a model of liver dysfunction. In this study, PAG activity was increased in kidney and duodenum, which could contribute significantly to systemic hyperammonaemia. PAG activity was increased in cortex and basal ganglia, which might be responsible for brain hyperammonemia and intra-mitochondrial ammonia levels derived from glutamine hydrolysis that are maintained because successful detoxification by glutamine-synthetase is precluded.
During the 2nd half of the 20th century, hyperam-monaemia was considered to be derived from urea breakdown by intestinal bacteria and the majority of treatments are targeted against bacteria-derived ammonia from the colon[1]. However, the hypothesis was not universally accepted[12]. Hyperammonaemia following portacaval shunting in rats has been found to be similar in germ-free as well as in non-germ-free animals [13,14], providing support to the concept that hyperammonaemia and encephalopathy could develop without participation of bacteria[15]. The highest hyperammonaemia has been found in portal-drained viscera and derived mainly from glutamine deamidation[16]. Hence, increased PAG activity in small intestine could explain these observations at least in part. Moreover, increased PAG in duodenum has been demonstrated in cirrhotic patients suffering from minimal hepatic encephalopathy[3].
Recently, renal ammoniagenesis has been implicated in several forms of hepatic encephalopathy. In patients suffering from hepatic encephalopathy due to variceal bleeding or overdose of diuretics, ammonia production from kidney seems to be the main factor involved in the development of hepatic encephalopathy[17]. Glutamine is filtered in the glomeruli and enters the lumen of the nephron. The filtered glutamine is reabsorbed in the proximal tubule where glutamine is deamidated by PAG. The main factor in the regulation of kidney PAG activity seems to be pH. During metabolic acidosis, PAG activity increases which induces higher excretion of ammonia[18]. In hepatic encephalopathy, metabolic alkalosis is observed more frequently than acidosis and increased PAG activity in kidney could be a protective mechanism. This PAG increase could be even more protective than the event associated with the systemic hyperammonaemia. However, this possibility needs to be explored in greater detail in future studies.
Ammonia reaching the brain can be detoxified to glutamine only in astrocytes due to the predominance of glutamine synthetase in these cells. Glutamine accumulation as a by-product of ammonia metabolism has been implicated in the pathogenesis of hepatic encephalopathy[19]. Glutamine is an osmotic amino acid and plays a major role in the regulation of cell volume. A raised peak of glutamine-glutamate/creatine ratio is a typical feature of brain spectroscopic magnetic resonance imaging in hepatic encephalopathy. However, glutamine synthetase has not been found to be increased in brain of portacaval shunted rats[20]. Indeed, administration of amino acid mixtures induces hyperammonaemia, raises glutamine peak in the brain and is associated with impairment in neuropsychological function[21]. Also, use of methionine-sulfoxamine blocks the activity of glutamine synthetase and improves abnormalities induced by ammonia, such as seizures or astrocytes swelling[22]. Nevertheless, accumulation of glutamine following treatment with drugs that are able to block N-methyl-D-aspartate (NMDA)-receptors has not been shown to be associated with neurological impairment[23]. Indeed, in astrocyte culture, glutamine metabolism is linked to free radical production and oxidative stress and this may represent a key mechanism in ammonia neurotoxicity [24]. Thus, glutamine accumulation in the brain is neither a pathological event per se nor a safe ammonia detoxification pathway. Glutamine accumulating in the astrocytes can be considered as a “Trojan horse” leading circuitously to neurological impairment. In cultured astrocytes, glutaminase inhibitors such as 6-diazo-5-oxo-L-norleucine (DON) induce a complete blockade of glutaminase activity and preempt free radical production and neurotoxicity induced by ammonia[25]. Most of the glutamine in astrocytes is metabolized by mitochondrial PAG[26]. Since ammonia induces free radical production, the PAG can be implicated in free radical production. The inhibition of this enzyme could be a new therapeutic target. However, DON has also been reported to inhibit γ-glutamyl-transpeptidase, increase glutamine release, inhibit transport of glutamine into cells and block the transport of glutamine into mitochondria. All these mechanisms could induce a decrease in the amount of glutamine available for hydrolysis[27]. The distribution of K-type PAG in the brain has been strongly debated. Some studies have reported that PAG is absent in astrocytes of cerebellum[28]. In the current study, PAG activity in non-synaptic mitochondria from the cerebellum showed the lowest activity in the brain and no differences were observed between PCA and sham-operated rats using immunoblotting. Thus, PAG could be detected in cerebellum, but at a low-level in comparison to other areas such as basal ganglia or cortex.
In summary, PAG is enhanced in the intestine and kidney of PCA rats and induces hyperammonaemia and hepatic encephalopathy. Furthermore, PAG activity and glutaminase content are increased in astrocytes from cortex and basal ganglia. Mitochondrial glutaminase activity in astrocytes could be implicated in the production of ammonia. The induction of the mitochondrial permeability transition and free radicals production as the end product of glutamine metabolism could be responsible at least in part for the pathogenic effect observed in hepatic encephalopathy. Hence, PAG might be a new therapeutic target in the management of hepatic encephalopathy. Further studies using PAG inhibitors or PAG knock-out mice help clarify the role of increased PAG expression in the pathophysiology of hepatic encephalopathy.
S- Editor Pan BR L- Editor Wang XL E- Editor Ma WH
| 1. | Sherlock S. Chronic portal systemic encephalopathy: update 1987. Gut. 1987;28:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Romero Gómez M, Bautista JD, Grande L, Ramos Guerrero RM, Sánchez Muñoz D. [New concepts in the physiopathology of hepatic encephalopathy and therapeutic prospects]. Gastroenterol Hepatol. 2004;27 Suppl 1:40-48. [PubMed] |
| 3. | Romero-Gómez M, Ramos-Guerrero R, Grande L, de Terán LC, Corpas R, Camacho I, Bautista JD. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol. 2004;41:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | James LA, Lunn PG, Elia M. Glutamine metabolism in the gastrointestinal tract of the rat assess by the relative activities of glutaminase (EC 3.5.1.2) and glutamine synthetase (EC 6.3.1.2). Br J Nutr. 1998;79:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | James LA, Lunn PG, Middleton S, Elia M. Distribution of glutaminase and glutamine synthetase activities in the human gastrointestinal tract. Clin Sci (Lond). 1998;94:313-319. [PubMed] |
| 6. | van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr. 2004;79:185-197. [PubMed] |
| 7. | Numata M. A modified technique to make a portacaval shunt in rats. Microsurgery. 1983;4:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Hermenegildo C, Monfort P, Felipo V. Activation of N-methyl-D-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology. 2000;31:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Lai JC, Clark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Methods Enzymol. 1979;55:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 207] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157357] [Article Influence: 3211.4] [Reference Citation Analysis (0)] |
| 11. | Felipo V, Miñana MD, Grisolía S. Long-term ingestion of ammonium increases acetylglutamate and urea levels without affecting the amount of carbamoyl-phosphate synthase. Eur J Biochem. 1988;176:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Shawcross D, Jalan R. Dispelling myths in the treatment of hepatic encephalopathy. Lancet. 2005;365:431-433. [PubMed] |
| 13. | Nance FC, Kline DG. Eck's fistula encephalopathy in germfree dogs. Ann Surg. 1971;174:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Warren KS, Newton WL. Portal and peripheral blood ammonia concentrations in germ-free and conventional guinea pigs. Am J Physiol. 1959;197:717-720. [PubMed] |
| 15. | Weber FL Jr, Veach GL. The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology. 1979;77:235-240. [PubMed] |
| 16. | Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond). 2004;106:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Curthoys NP. Role of mitochondrial glutaminase in rat renal glutamine metabolism. J Nutr. 2001;131:2491S-2495S; discussion 2496S-2497S;. [PubMed] |
| 19. | Albrecht J, Dolińska M. Glutamine as a pathogenic factor in hepatic encephalopathy. J Neurosci Res. 2001;65:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Desjardins P, Rao KV, Michalak A, Rose C, Butterworth RF. Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis. 1999;14:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Balata S, Olde Damink SW, Ferguson K, Marshall I, Hayes PC, Deutz NE, Williams R, Wardlaw J, Jalan R. Induced hyperammonemia alters neuropsychology, brain MR spectroscopy and magnetization transfer in cirrhosis. Hepatology. 2003;37:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Rama Rao KV, Jayakumar AR, Norenberg MD. Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int. 2003;43:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sánchez-Perez AM, Felipo V. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Jayakumar AR, Rama Rao KV, Schousboe A, Norenberg MD. Glutamine-induced free radical production in cultured astrocytes. Glia. 2004;46:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Norenberg MD, Rama Rao KV, Jayakumar AR. Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr. 2004;36:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Sonnewald U, Therrien G, Butterworth RF. Portacaval anastomosis results in altered neuron--astrocytic metabolic trafficking of amino acids: evidence from 13C-NMR studies. J Neurochem. 1996;67:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Rama Rao KV, Jayakumar AR, Norenberg MD. Differential response of glutamine in cultured neurons and astrocytes. J Neurosci Res. 2005;79:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88:1137-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |