Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2394
Revised: November 2, 2005
Accepted: November 18, 2005
Published online: April 21, 2006
AIM: To investigate the hepatocytic differentiation of mesenchymal stem cells (MSCs) in co-cultures with fetal liver cells (FLC) and the possibility to expand differentia-ted hepatocytic cells.
METHODS: MSCs were marked with green fluorescent protein (GFP) by retroviral gene transduction. Clonal marked MSCs were either cultured under liver stimulating conditions using fibronectin-coated culture dishes and medium supplemented with stem cell factor (SCF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), and fibroblast growth factor 4 (FGF-4) alone, or in presence of freshly isolated FLC. Cells in co-cultures were harvested, and GFP+ or GFP- cells were separated using fluorescence activated cell sorting. Reverse transcription-polymerase chain reaction (RT-PCR) for the liver specific markers cytokeratin-18 (CK-18), albumin, and alpha-fetoprotein (AFP) was performed in different cell populations.
RESULTS: Under the specified culture conditions, rat MSCs co-cultured with FLC expressed albumin, CK-18, and AFP-RNA over two weeks. At wk 3, MSCs lost hepatocytic gene expression, probably due to overgrowth of the cocultured FLC. FLC also showed a stable liver specific gene expression in the co-cultures and a very high growth potential.
CONCLUSION: The rat MSCs from bone marrow can differentiate hepatocytic cells in the presence of FLC in vitro and the presence of MSCs in co-cultures also provides a beneficial environment for expansion and diffe-rentiation of FLC.
- Citation: Lange C, Bruns H, Kluth D, Zander AR, Fiegel HC. Hepatocytic differentiation of mesenchymal stem cells in cocultures with fetal liver cells. World J Gastroenterol 2006; 12(15): 2394-2397
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2394.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2394
The existence of putative liver stem cells in the bone marrow was first described by Petersen et al[1] who showed that bone marrow cells transplanted into lethally irradiated mice could engraft in the recipient,s liver and differentiate into hepatic stem cells (oval cells) or hepatocytes. First in vitro data suggest that several types of bone marrow cells / stem cells can differentiate towards hepatocytic cells under the appropriate culture conditions[2-5]. Oh et al[6] found that the liver specific proteins alpha-feto protein (AFP) and albumin are expressed in cultures of unsorted rat bone marrow cells after 21 d. Furthermore, several recent in vitro studies indicate the possibility of hepatocytic differentiation of MSCs in vitro[7-9]. Kang et al[7] reported that MSCs from the rat bone marrow can express the liver specific marker AFP, and produce albumin and urea in vitro when cultured in the presence of cytokines, fibroblast-growth factor (FGF-4) and hepatocyte growth factor (HGF). Hong et al[8] demonstrated that the liver specific genes cytokeratin (CK) 18, AFP, and albumin are expressed in cultures of human umbilical cord-blood derived MSCs. Furthermore, Lee et al[9] also showed liver specific functions of cytochrome P450 activity, urea and albumin-production, and glycogen storage in hepatocytic differentiated umbilical cord blood-derived MSC cultures. We had also shown that rat MSCs have a differentiation potential towards hepatocytic cells when co-cultured with adult rat liver cells[10,11]. The fetal milieu, however, might permit a more suitable environment for rapid MSC-maturation into hepatocytic cells.
In this study, we investigated the potential of rat me-senchymal stem cells derived from adult bone marrow to differentiate into hepatic cells in vitro under the direct influence of fetal liver cells for the initiation of liver specific gene expression.
Isolation, transduction and cloning of rat mesenchymal stem cells were carried out as described previously[10]. Rat embryos of embryonal/ fetal day (ED) 16 were harvested by section after the mother was sacrificed by an overdose of ether-anesthesia. Fetuses were dissected under the dissection microscope. Fetal livers were harvested and liver cells were isolated by a collagenase digestion using collagenase type IV (Sigma, St. Louis, USA) as described previously[12]. A Percoll (P = 1 124 g/mL, Biochrom, Berlin, Germany) gradient centrifugation was used to enrich the viable cell fraction. The Percoll concentration used was 76%. Magnetic cell sorting was used for the removal of fetal OX-43 and OX-44 positive hematopoietic cells from the freshly isolated cells as described elsewhere[13]. In brief, isloated cells were marked with mAb OX-43 and mAb OX-44 (both Serotec, Eching, Germany). Unbound antibodies were removed and secondary marking was done with goat-anti-mouse IgG bound magnetic microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). Positive cells were absorbed in a magnetic field by Vario-MACS columns, the depleted cell fraction was collected in a tube.
Cultures were performed according to our protocol published previously[10]. In brief, cells were seeded on culture wells coated with fibronectin (Sigma) on 24-well plates (Greiner). rMSCs (9 ×104) per well were seeded for MSC-controls, or 6×104 fetal liver cells per well for controls. For co-cultures of rMSCs with fetal rat liver cells, 9×104 rMSCs per well were preceded in 24-well plates for 2 - 3 h. Then, 6 × 104 fetal rat liver cells per well were added to the cultures. Medium was changed twice a week. Stem Span SFEM (Stem Cell Technologies, St. Katherinen, Germany) medium was supplemented with penicillin/streptomycin (Gibco), dexamethasone (Sigma), 100 ng/mL human recombinant stem cell factor (SCF), 20 ng/mL hepatocyte growth factor (HGF) (both Immunotools, Friesoythe, Germany), 50 ng/mL epidermal growth factor (EGF), and 10 ng/mL fibroblast growth factor-4 (FGF-4) (both R&D, Wiesbaden, Germany). Cultures were analyzed on d 7, 14, and 21.
After the culture period, cells from co-cultures were trypsinized, counted with trypan blue, and re-suspended in 3mL PBS. To get single cell suspension, cells were filtered through a 35 µm filter (BD, Heidelberg, Germany). Cells were sorted using the FACS AriaTM Cell Sorter (BD) into GFP-positive (GFP+) or GFP-negative (GFP-) cells, focussing on the highest possible purity of GFP+ cells.
RNA was extracted using the Invisorb Spin Cell-RNATM Mini-kit (Invitek, Berlin, Germany) according to the manufacturer’s instructions and stored at -80°C. Reverse transcription (RT) of extracted RNA was performed using the bulk first-strand c-DNA synthesis kit (Amersham, Freiburg, Germany). The cDNA was stored at -20°C. For PCR, 5 µL of cDNA-template was mixed with 2.5 µL of 10 × PCR-buffer, 0.5 µL of 10 mmol dNTP´s, 0.5 µL of each primer (50 ng/µL), and 0.5 µL of polymerase (Ampli-Taq., Gibco) in a total volume of 25 µL for each probe. PCR was carried out in a programmable Biometra Uno-Thermobloc (Biometra, Göttingen, Germany) using the primers for albumin, AFP, and CK-18 as described previously[10]. Negative controls were performed for each set of primers. Samples were analyzed on 1% agarose gels. The size of the PCR-fragments was estimated using the 100-base-pair ladder (Gibco BRL).
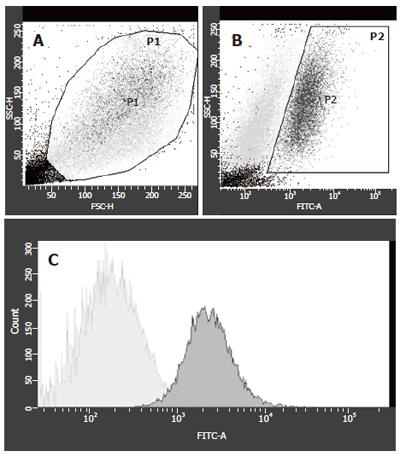
The sorting strategy concentrated on maximal purity of GFP+ cells to exclude contamination with FLC-derived RNA. A typical example for FACS 1 wk after co-culture is shown in Figure 1. The viable cells were gated according to forward and side scatter properties (Figure 1A). Two populations varied in size and granulation. GFP-expression of the gated cells was investigated in two populations, differing in granulation (Figure 1B) and gated as P2 cells. From these P2-cells, the contaminating GFP- cells were sorted out (Figure 1C). All non-P1, non-P2 and non-GFP cells were collected as GFP- cells. When the number of viable cells (gate P1) was set as 100%, the fraction of GFP positive cells decreased from 24.2 % of viable cells at wk 1 to 1.3 % of viable cells at wk 3 (Table 1). This was also consistent with the morphological findings in the co-cultures, where a massive overgrowth of the adherent MSCs to the fetal liver cells was observed.
| Wk 1 | Wk 2 | Wk 3 | |
| Viable Cells | 70.2 | 79.1 | 77 |
| GFP-positive cells | 34.5 | 5.6 | 1.7 |
| GFP-negative cells | 65.5 | 94.4 | 98.3 |
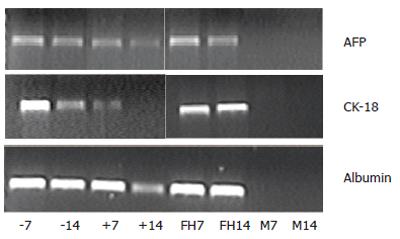
Glyceraldehyde phosphate dehydrogenase (GAPDH) expression in cultured rMSCs, fetal liver cells, or GFP+ and GFP- FACS cells was equal at all time points (data not shown). Cultured rMSCs showed no expression of AFP, CK-18, or albumin on d 7 or 14 (Figure 2, lanes M7 and M14). Cultured fetal liver cells showed expression of AFP, CK-18 and albumin over the two wk culture period (Figure 2, lanes FH7 and FH14). The GFP+ cells (Figure 2, lanes +7 and +14) showed a strong expression of albumin and AFP, and a declining expression of CK-18. The GFP- cells (Figure 2, lanes -7 and -14) showed a stable expression of AFP, CK-18, and albumin. Negative controls without template were negative at all time points and probes (data not shown). On d 21, no liver specific genes were detected in the GFP+ cells (data not shown) probably due to the small cell number.
In this study, cloned GFP+ rMSCs from passage ≥ 9 were used for the differentiation analysis towards liver cells. For liver specific differentiation, cells were cultured on a fibronectin matrix in serum free medium containing the liver growth factors: HGF, EGF, FGF-4, and the stem cell growth factor: SCF. The impact of FLC on hepatic differentiation was assessed in co-cultures of GFP+ rMSCs with freshly isolated fetal rat liver cells (GFP-negative), and compared to cultures with rMSCs or FLC alone. For gene-expression analysis, cells from co-cultures were separated in GFP+ or GFP- cells by fluorescence-activated cell sorting before RT-PCR analysis.
Albumin is a typical marker of mature hepatocytes, whereas CK18 is expressed in several liver cell types, including biliary epithelial cells and hepatic oval cells[14]. AFP in the liver is a marker of immature (e.g. fetal) liver cells or oval cells in the adult liver[15]. Our data indicate that rMSCs possess a differentiation potential towards hepatocytic cells in vitro. Expression of the liver specific genes CK-18, albumin, and AFP was demonstrated in GFP+ cells of the cocultures for two weeks. Additionally, we found that liver specific gene expression in mesenchymal stem cells was induced by the coculture with isolated FLC. Cultured rMSCs alone did not express any of the liver specific genes stu-died in the presence of fibronectin (FN)-coating and liver-differentiation stimulating growth factors. Avital et al[16] have highlighted the effects of co-cultured hepatocytes (separated by a PTFE-membrane) for β2m-negative thy1-positive stem cells from rat bone marrow expressing the liver marker albumin[16]. An important influence of hepatocytes on the differentiation of stem cell-enriched bone marrow has also been highlighted by Okumoto et al[17] who found that MSCs-enriched bone marrow stem cells cultured in the presence of HGF and fetal bovine serum (FBS) express the markers: hepatic nuclear factor 1 (HNF1-α) and CK-8 only after 7 d. In co-cultures with hepatocytes separated by a semi-permeable membrane the stem cells additionally express the liver specific markers: AFP and albumin. We found a beneficial effect for differentiation and growth of the co-cultured fetal liver cells (GFP-), which also showed a stable liver specific gene expression and viability over the whole observation period. This is consistent with findings of Hoppo et al [18] who showed a positive influence of MSCs on cultured endodermal fetal liver cells of the mouse in culture. However, the FLC tended to overgrow the adherent layer of MSCs at the end of the culture period. Thus, we could not detect any liver specific gene expression in the few remaining GFP+ MSC-derived cells in the co-cultures at wk 3. Our results support the notion of a faster differentiation of MSCs into hepatocytic cells under the influence of fetal liver cells compared to adult hepatocytes. The growth potential of MSCs seems to be impaired by the huge overgrowth of FLC, thus limi-ting the desired expansion of differentiated MSCs. Based on the declined GFP-positive fraction of cells and the decreased intensity of semi-quantitative PCR-signals over the experimental time of 3 wk, differentiation of rMSCs into hepatocytic cells could be suggested. However, fusion events can not be ruled out by our data.
Our in vitro data indicate that mesenchymal stem cells from rat bone marrow possess a differentiation capacity towards hepatocytic cells in vitro. Furthermore, we showed a strong influence of co-cultures with fetal liver cells on the induction of liver specific gene expression of cultured stem cells. However, fetal liver cells tended to overgrow the adherent MSCs in the co-cultures.
In conclusion, the co-culture of MSCs and FLC is a feasible culture model which might provide more insights into the relationship between fetal hepatopoietic cells during liver development.
The authors thank Arne Düsedau at Heinrich Pette Institute of the University of Hamburg for the FAC-sorting, Mrs. B. Roth at Department of Pediatric Surgery for technical assistance and the foundation “Deutsche José-Carreras-Leukaemiestiftung” for financing technical equipment. The rats were purchased from Hannover Medical School Animal Center with support of Marc Dahlke.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 2. | Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 6. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Kang XQ, Zang WJ, Song TS, Xu XL, Yu XJ, Li DL, Meng KW, Wu SL, Zhao ZY. Rat bone marrow mesenchymal stem cells differentiate into hepatocytes in vitro. World J Gastroenterol. 2005;11:3479-3484. [PubMed] |
| 8. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 659] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol. 2005;11:4497-4504. [PubMed] |
| 11. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc. 2005;37:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Fiegel HC, Park JJ, Lioznov MV, Martin A, Jaeschke-Melli S, Kaufmann PM, Fehse B, Zander AR, Kluth D. Characterization of cell types during rat liver development. Hepatology. 2003;37:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Fiegel HC, Kluth J, Lioznov MV, Holzhüter S, Fehse B, Zander AR, Kluth D. Hepatic lineages isolated from developing rat liver show different ways of maturation. Biochem Biophys Res Commun. 2003;305:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Brill S, Holst P, Sigal S, Zvibel I, Fiorino A, Ochs A, Somasundaran U, Reid LM. Hepatic progenitor populations in embryonic, neonatal, and adult liver. Proc Soc Exp Biol Med. 1993;204:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Okumoto K, Saito T, Hattori E, Ito JI, Adachi T, Takeda T, Sugahara K, Watanabe H, Saito K, Togashi H. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem Biophys Res Commun. 2003;304:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, Naito M, Machimoto T, Ikai I. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology. 2004;39:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |