Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2351
Revised: November 2, 2005
Accepted: November 10, 2005
Published online: April 21, 2006
AIM: To investigate the effects of verapamil on nitric oxide (NO) synthesis in a portal vein-ligated rat model.
METHODS: Systemic and splanchnic hemodynamics were measured by radiolabeled microspheres in portal hypertensive rats after acute administration of verapamil (2 mg/kg) on chronic treatment with Nw–nitro-L-arginine (NNA)(80 mg/kg) and/or indomethacin (2 mg/kg) .
RESULTS: Verapamil (2 mg/kg) caused a marked fall in both arterial pressure and cardiac output accompanied by an insignificant change in the portal pressure and no change in portal venous inflow. This result suggested that verapamil did not cause a reduction in portal vascular resistance of portal hypertensive rats, which was similar between Nw- nitro–L-arginine-treated and indomethacin-treated groups.
CONCLUSION: In portal hypertensive rats pretreated with NNA and/or indomethacin, acute verapamil administration can not reduce the portal pressure, suggesting that NO and prostaglandin play an important role in the pathogenesis of splanchnic arterial vasodilation in portal hypertension.
- Citation: Lay CS, May C, Lee FY, Tsai YT, Lee SD, Chien S, Sinchon S. Effect of verapamil on nitric oxide synthase in a portal vein-ligated rat model: Role of prostaglandin. World J Gastroenterol 2006; 12(15): 2351-2356
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2351
Verapamil, a calcium entry blocker, in addition to its inhibiting action on the contraction of rat portal vein[1,2], can decrease hepatic venous pressure gradient[3,4] and is proposed as a drug in the treatment of variceal bleeding in patients with HBsAg-positive cirrhosis[4,5]. It was reported that verapamil reduces the intrahepatic shunting in cirrhotic rat- perfused liver[6]. Furthermore, verapamil can also reverse the altered microvascular exchange caused by sinusoidal capillarization in cirrhotic rats when given acutely or chronically[6,7]. However, studies testing verapamil, nifedipine and nicardipine in patients with cirrhosis have failed to show any reduction in hepatic vein pressure gradient, but increase in portal collateral blood flow, an effect that may be dangerous in patients with esophageal varices[8-10]. So far, the mechanism responsible for the effect of verapamil on splanchnic circulation in the rat model of pre-hepatic portal hypertension is still unknown.
Nitric oxide (NO) and prostacyclin (PGI2) are endogenous vasodilators synthesized by the vascular endothelium[11-13]. In fact, both can modulate the mesenteric vascular tone and are important contributors to splanchnic arterial vasodilation in portal hypertensive rats[12,13]. In addition, some investigators have reported that NO plays a major role in modulating collateral vascular resistance[14-16]. Increased PGI2 activities have been observed in the splanchnic vascular bed of portal hypertensive rat model[17-19]. However, it is unknown whether both vasodilators modify the response of splanchnic arterial vasodilation to verapamil in portal hypertensive rats. Thus, we aimed to identify any unique advantageous or deleterious haemodynamic effect of acute verapamil on nitric oxide synthese in a pre-hepatic portal hypertensive rat model. In addition, the influence of NO and prostaglandin on the responsiveness of splanchnic arterial vasodilation to verapamil was also evaluated.
Male Sprague-Dawley rats weighing 280-340 g at the time of surgery were used for experiments. The rats were housed in a plastic cage and allowed free access to food and water. All rats were fasted for 12 h before operation. In all experiments, the investigators followed the American Physiological Society Guiding Principles for the Care and Use of Laboratory Animals.
A prehepatic portal hypertensive animal model was induced by partial portal vein ligation (PVL) as previously described[20]. Anesthesia was performed with ketamine HCl (100 mg/kg body weight, intramuscularly). In brief, the portal vein was isolated and a 3-0 silk ligature was tied around the portal vein and a 20-gauge blunt-tipped needle. The needle was then removed and the vein allowed to reexpand. A second loose ligature was left around the portal vein with the 2 ends of the ligature placed on each side in the abdominal cavity. The abdomen was then closed and the animal was allowed to recover. Perfusion studies were performed on overnight-fasted rats 10-13 d after the operation, at which time an extensive collateralization of the portal system was fully established.
Rats with a ligated portal vein were divided into three groups. In each group of PVL rats, verapamil (Isoptin Knoll AG, Ludwigshafen, Germany) was given at a dose of 2 mg/kg parenterally, which decreased the mean arterial pressure (MAP) by more than 10%. One group of sham-operated rats received sterile saline (1 mL/kg) only. A group of PVL rats were also studied after the administration of sterile saline (1 mL/kg) alone to ensure that the hyperdynamic state could be achieved. Pressure dose-response curves were constructed for each group of rats 15 min after the administration of each dose. Seven doses were used at the concentration ranging from 0.02 to 2.0 mg/kg body weight. Verapamil was administered through a venous catheter. In the first group, sham-operated and PVL rats were treated daily following the ligation with one ip injection of Nw–nitro-L-arginine (NNA) (80 mg/kg). In the second group, sham-operated and PVL rats were treated daily following the ligation with one ip injection of indomethacin (INDO) (2 mg/kg). In the third group, sham-operated and PVL rats were treated daily following the ligation with ip injection of both NNA (80 mg/kg) and indomethacin (2 mg/kg).
All animals were anaesthetized with pentobarbital (50 mg/kg) and fastened on an animal board in dorsal recumbency. Both femoral vein and artery were cannulated with a polyethylene catheter (PE-50). The mean arterial blood pressure (MAP) was measured with a strain-gauge transducer (Statham P23 Db) connected to the femoral artery cannula and recorded continuously on a Grass polygraph (Grass Apparatus, Quincy, MA, USA). The skin over the left upper abdominal quandrant was shaved, a small (1.0 cm-2.0 cm) parasagittal incision was made at the level of the left anterior axillary line and the muscles were gently separated with forceps. The spleen was exposed by pulling it away from the perisplenic fat and then covered with a piece of warm Ringer-lactate moist gauze. Special care was taken to avoid any unnecessary manipulation of the spleen. The jejunal vein was cannulated with a PE-50 catheter for the continuous monitoring of portal pressure. Catheters were also placed in the left femoral artery and right carotid artery. The right carotid artery catheter was carefully advanced into the left ventricle under continuous pressure. Heart rate measurement was used to radiolabel the microsphere injection for the determination of portal venous inflow (PVI). All catheters were connected to Statham P-23-Db strain gauge transducers (Statham), and continuous pressure recordings of left ventricular and portal pressures (PP) were printed on a Grass model ID inscription recorder (Grass, Quincy, MA, USA). Baseline pressure and heart rate measurement were recorded in each animal.
Splanchnic organ blood flow and cardiac output were determined twice (before and 15 min after administration of the drug ) according to the reference sample method with intracardiac injection of isotope-labelled microspheres (15±3 μm in diameter)[21].「57Co」 and「113Sn」-labelled microspheres (New England Nuclear, Boston, MA, USA) suspended in Tween 80 (0.05%) were used for the first or second injection. Approximately 180 000「57Co」 and 「113Sn」-labelled microspheres were aspirated into plastic 1.0 mL syringes (volume 0.3-0.4 mL) and mixed in a vortex for 5 min. An additional 0.2 mL sample containing approximately 30 000「46Sc」-labelled microspheres was also placed into a 1.0 mL plastic syringe until injection.
Ten seconds after withdrawal of a blood sample from the left femoral artery for reference, microspheres were injected into the left ventricle over 10-15 s. Catheters were flushed with 0.2 Ml of 0.9% NaCl. Blood was withdrawn from the left femoral artery over 90 s at an approximate rate of 1 mL/min using a Harvard pump (Harvard Apparatus, Millis, MA, USA). Once withdrawal was complete, a volume of 0.9% NaC1 equal to the sample volume was withdrawn and reinfused, arterial blood pressure was monitored to ensure stability during microsphere distribution. As soon as the spleen was exposed, the「46Sc」-labelled microspheres were injected through a 23-gauge needle into the splenic pulp over a period of 20 s. The animals were then killed with an injection of bolus of saturated KC1 into the carotid artery catheter.
The liver, lungs, stomach, intestine, spleen, pancreas, mesentery and kidneys were dissected and weighed. The radioactivity of the organs was determined in a scintillation counter (Packard, Downers Grove, IL, USA) with an energy window set at 50-200 KeV for 57Co, at 300-500 KeV for 113Sn and at 800-1200 KeV for 46Sc, respectively. At least 300 microspheres were trapped in the reference sample and organs to ensure validity of the measurement. The error in the measurement of radioactivity induced by spillover of each radioactive microsphere channel was corrected using 57Co, 113Sn and 46Sc standards. Each standard was checked by a multichannel analyzer (Series 35 PLUS). Adequate microsphere mixing was assumed at a difference < 20% between the left and right kidneys. Any animal not meeting this requirement was rejected from the analysis. Cardiac output was calculated according to the following formula:
Cardiac output (mL/min) = (injected radioactivity (cpm) × reference sample blood flow (mL/min)) / reference sample radioactivity (cpm).
Injected radioactivity was calculated on the basis of the difference between the initial and residual radioactivity in the syringe. Organ blood flow was calculated as follows:
Organ blood flow (mL/min) = (organ radioactivity (cpm) × reference sample blood flow (mL/min)) / reference sample radioactivity (cpm).
Vascular resistance of different organs in the portal venous system was calculated according to the following formula:
Resistance (dyn.s.cm-5×105) = (mean arterial pressure (mmHg) - portal venous pressure (mmHg)) × 80/organ blood flow (mL/min)
Portal tributary blood flow was calculated as the sum of the blood flow in the spleen, stomach, colon, mesentery and pancreas. The hepatic arterial blood flow was taken to be equal to the liver blood flow.
Portal systemic shunt (PSS) % was calculated as: [ct/min in lung/ct/min in (lung + liver)] x 100
Verapamil, NNA, INDO, and reagents for preparing Krebs’ solution were purchased from Sigma. All solutions were freshly prepared on the day the experiment was conducted.
Results were expressed as mean ± SD. The concentration of verapamil at 50% of the maximal response (EC50) in each preparation was calculated from sigmoid logistic curves and expressed as negative log molar (-log mol/L). Statistical analyses were performed by the paired or unpaired Student’s t test and one-way analysis of variance with Tukey’s test when appropriate. P<0.05 was considered statistically significant.
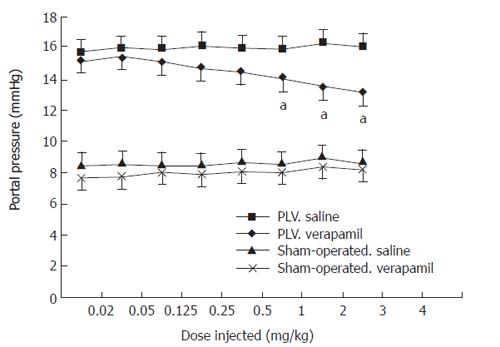
No differences were observed in body weights, mean arterial pressures and heart rates among the three groups (Table 1). The PVL rats exhibited a hyperdynamic state of splanchnic circulation with a high cardiac index. The pressure-dose-response curves of the sham-operated and PVL rats after acute verapamil administration in different groups were similar to the curves previously reported by us[20](Figure 1).
| Groups | n | Body weight | Mean arterial | Heart rate |
| ( g ) | pressure(mmHg) | (beat/min) | ||
| Vehicle | 8 | 326.7 ± 10.6 | 98.6 ± 3.6 | 296 ± 18 |
| Verapamil | 8 | 332.6 ± 11.8 | 96.5 ± 4.8 | 320 ± 16 |
| Verapamil+vehicle | 8 | 321.5 ± 9.8 | 99.5 ± 5.2 | 318 ± 20 |
| Verapamil+NNA | 8 | 330.8 ± 9.6 | 97.4 ± 3.8 | 317 ± 17 |
| Verapamil+INDO | 7 | 327.8 ± 8.5 | 94.2 ± 6.2 | 328 ± 14 |
| Verapamil+ INDO+NNA | 8 | 319.8 ± 7.9 | 96.2 ± 4.8 | 308 ± 15 |
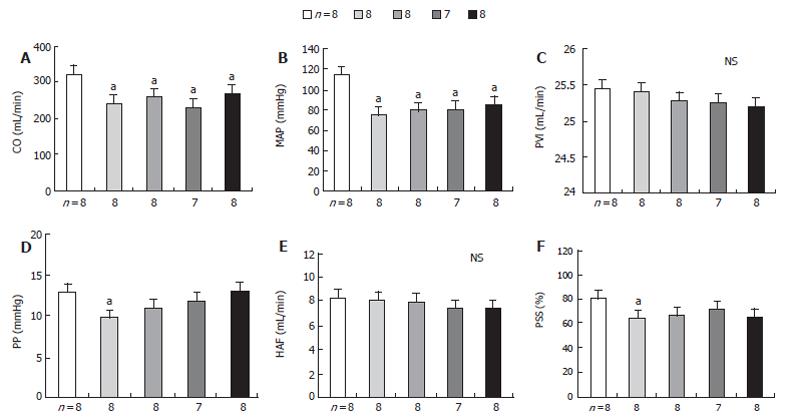
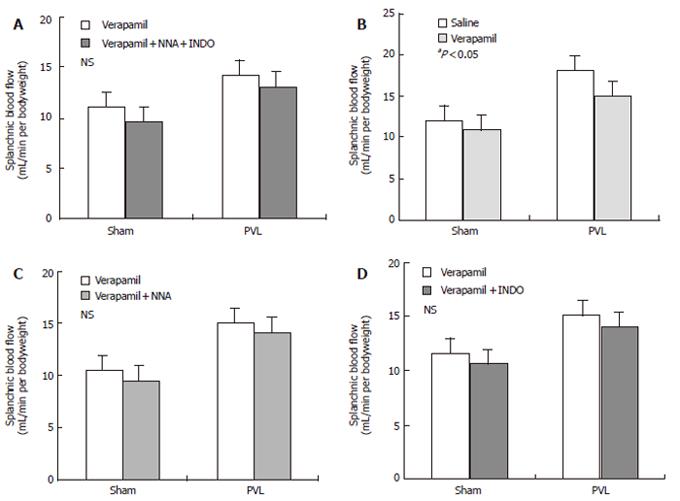
No significant change of the portal pressure (PP) was noted when verapamil (2 mg/kg) was given to PVL rats by early chronic administration of NNA, INDO, or both in all three groups. The results of this study also indicated that acute verapamil administration could reduce the PP in different study groups of PVL rats in a hyperdynamic state. In all three groups of rats, the mean arterial pressure (MAP) significantly decreased following administration of verapamil compared to the control group. In contrast, the portal pressure, portal venous inflow (PVI) and hepatic arterial flow (HAF) were maintained, while cardiac output (CO ) decreased significantly compared to the control group (Table 2, Figure 2). Thus, the effect of verapamil on chronic treatment with NNA (80 mg/kg), INDO (2 mg/kg), and both on splanchnic organ blood flow in sham-operated and PVL rats after 15 d from the control are shown in Figure 3. At a dose of 2 mg/mL verapamil, there was also no significant decrease in total peripheral resistance (TPR), splanchnic arterial resistance (SAR) and portal venous resistance (PVR) in all three groups of PVL rats (Table 3).
| Verapamil | Verapamil+NNA | Verapamil+INDO | Verapamil+NNA+INDO | |||||
| (n = 8) | (n = 8) | (n = 7) | (n = 8) | |||||
| Before | After | Before | After | Before | After | Before | After | |
| CI[mL/(min.kg)] | 423 ± 38 | 298 ± 42a | 425 ± 41 | 312 ± 48a | 424 ± 39 | 332 ± 51a | 426 ± 40 | 326 ± 49a |
| MAP(mmHg) | 105 ± 5 | 96 ± 6a | 106 ± 6 | 99 ± 7a | 107 ± 4 | 98 ± 7a | 106 ± 8 | 100 ± 7a |
| PP(mmHg) | 15.0 ± 1.6 | 10.8±1.9a | 14.3 ± 1.8 | 13.8 ± 2.1 | 15.2 ± 1.9 | 14.5 ± 2.4 | 15.1 ± 1.6 | 14.6 ± 1.4 |
| PVI(mL/min) | 27 ± 2.8 | 26 ± 3.2 | 26 ± 2.7 | 25 ± 3.4 | 28 ± 3.1 | 27 ± 3.5 | 27 ± 3.2 | 26 ± 3.9 |
| HAF(mL/min) | 7 ± 1.6 | 8 ± 1.8 | 8 ± 1.8 | 9 ± 2.2 | 7 ± 1.9 | 8 ± 2.2 | 9 ± 2.0 | 9 ± 2.8 |
| PSS(%) | 81.7 ± 7.2 | 63.6 ± 8.2a | 80.2 ± 8.3 | 76.5 ± 9.2 | 81.2 ± 6.2 | 77 ± 7.8 | 80.5 ± 6.6 | 76.2 ± 9.5 |
| Verapamil | Verapamil+NNA | Verapamil+INDO | Verapamil+NNA+INDO | |||||
| (n = 8) | (n = 8) | (n = 7) | (n = 8) | |||||
| Before | After | Before | After | Before | After | Before | After | |
| TPR (dyh.s.cm-5× 104) | 8.96 ± 0.87 | 6.45 ± 0.78 a | 8.84 ± 0.72 | 8.47 ± 0.98 | 8.76 ± 0.88 | 8.52 ± 0.91 | 8.78 ± 0.93 | 8.58 ± 0.89 |
| SAR (dyh.s.cm-5× 105) | 4.38 ± 0.72 | 3.16 ± 0.62 a | 4.51 ± 0.89 | 4.18 ± 0.71 | 4.45 ± 0.92 | 4.20 ± 0.84 | 4.46 ± 0.84 | 4.22 ± 0.94 |
| PVR (dyh.s.cm-5 × 104) | 7.49 ± 0.68 | 6.12 ± 0.58 a | 7.46 ± 0.72 | 7.23 ± 0.67 | 7.54 ± 0.97 | 7.41 ± 0.95 | 7.46 ± 0.65 | 6.95 ± 0.87 |
After PVL rats were pretreated with either NNA or indomethacin for 15 d, acute verapamil (2 mg/kg) administration did not induce any significant reduction in the portal-systemic shunting. Furthermore, the portal pressure in both NNA- and indomethacin-pretreated PVL rats was also not significantly different from that in the control group. The portal pressure reduction was not correlated with portal-systemic shunting reduction in either NNA- or indomethacin-pretreated PVL rats (r = 0.05, NS; r = 0.03, NS).
The results of the present study showed that verapamil administration did not favorably influence splanchnic arterial vasodilation in portal vein-ligated rats pretreated with NNA and indomethacin as expected.
These findings are not consistent with those of a previous study in rats with carbon tetrachloride-induced cirrhosis[6], in which rat model it was demonstrated that verapamil reduces hepatic resistance, exerts beneficial effect on the hepatic microvascular exchange, improves liver function and reduces portal pressure if chronically administered. It was reported that verapamil is a useful therapeutic agent for patients with cirrhosis[4]. In a small group of patients, Freeman et al[3] demonstrated that verapamil administered intravenously, decreases the hepatic venous pressure gradient (HVPG). Furthermore, we have obtained similar results in patients with postnecrotic cirrhosis after both acute and chronic administration of verapamil[4,5].
The present study failed to document any beneficial effect of verapamil in portal hypertensive rats pretreated with NNA and indomethacin. This might not be due to a low dose or a poor absorption of verapamil, as documented by the dose response curve which was well within the therapeutic range. Pharmacodynamic efficacy was also manifested by the observed systemic effects of verapamil, including increased HR and peripheral vasodilation as well as decreased arterial pressure and SVR. Verapamil did not exert any noticeable effect on portal pressure, PVI and HAF. Accordingly, the hepatic vascular resistance did not decrease in our study. Because of this lack of effect on the splanchnic and hepatic arterial vasodilation, verapamil failed to influence liver perfusion. Thus, our results do not support the use of verapamil in the treatment of cirrhosis and portal hypertension pretreated with NNA and indomethacin. Further evidence against the use of verapamil in portal hypertension has been demonstrated by Narasa et al[8] who in accordance with our findings have failed to document any decrease in portal pressure following verapamil administration in portal hypertensive patients.
The reason for the discrepancies between the current results and previous studies is not clear. It may be related to the fact that our rat model was produced by portal vein ligation, causing presinusoidal portal hypertension, and these rats were pretreated with NNA and indomethacin. It is also possible that in this kind of animal model, verapamil cannot reduce portal pressure by decreasing intrahepatic portal resistance, and improve hepatic function by affecting the apparent hepatic blood flow[22]. So far, the effect of verapamil on rat hepatic stellate cells is still unknown[23]. The structural changes have not been observed and within the liver the hepatic microcirculation cannot be pharmacologically altered because verapamil has a high first-pass effect[1,24]. However, we have previously documented in a similar model of portal hypertensive rats that the hepatic vascular resistance and portal pressure can be lowered by verapamil[4]. Although animal experiments have demonstrated that verapamil can produce a complex interplay of alterations in preload (splanchnic venodilatation), afterload and myocardial contractility by inhibiting the constrictor responses of the splanchnic capacitance vessels of small resistant arterioles and arteries to the sympathetic nervous outflow[1,6,7], this finding indicates that the effect of verapamil on splanchnic arterial vasodilation can be pharmacologically modified by pretreatment with NNA and indomethacin.
Nitric oxide and prostaglandin are endogenous vasodilators produced by vascular endothelial cells[11-15]. Non-specific inhibition of NO synthesis can restore mesenteric vascular responsiveness[25] and normalize splanchnic vascular resistance[26], thus ameliorating portal-systemic shunting and hyperdynamic circulation[27,28]. Both NO and prostaglandin participate in the regulation of vascular tone in different vascular beds of normal and portal hypertensive animals[15-18]. It has been shown that in portal hypertensive rats, inhibition of nitric oxide synthesis increases not only intrahepatic and splanchnic vascular resistance but also portal-collateral resistance[11,15,29]. Recently, increased prostacyclin (PGI 2) activities have been observed in systemic circulation and portal vein segments of portal hypertensive rats[18]. In addition, prostaglandin is involved in the modulation of collateral vascular tone in portal hypertensive rats and can modify the vasoconstrictive effect of vasopressin[25]. Nitric oxide and PGI 2 produce relaxation of the vascular smooth muscle by different mechanisms. Nitric oxide works by activating guanylate cyclase which increases the intracellular levels of guanosine 3, 5-cyclic monophosphate[12].
In conclusion, acute verapamil administration does not increase the splanchnic arterial vasodilation in pre-hepatic portal hypertensive rats pretreated with NNA and /or indomethacin. In conjunction with our previous reports[20], these findings indicate that both NO and prostaglandin can modulate the splanchnic vascular response to verapamil and may therefore participate in the development and maintenance of portal hypertension.
The authors gratefully acknowledge Shwu-Ling Wu for her excellent technical skills.
S- Editor Guo SY L- Editor Wang XL E- Editor Ma WH
| 1. | Singh BN, Ellrodt G, Peter CT. Verapamil: a review of its pharmacological properties and therapeutic use. Drugs. 1978;15:169-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 304] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Dacquet C, Mironneau C, Mironneau J. Effects of calcium entry blockers on calcium-dependent contractions of rat portal vein. Br J Pharmacol. 1987;92:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Freeman JG, Barton JR, Record CO. Effect of isosorbide dinitrate, verapamil, and labetalol on portal pressure in cirrhosis. Br Med J (Clin Res Ed). 1985;291:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 4. | Kong CW, Lay CS, Tsai YT, Yeh CL, Lai KH, Lee SD, Lo KJ, Chiang BN. The hemodynamic effect of verapamil on portal hypertension in patients with postnecrotic cirrhosis. Hepatology. 1986;6:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lay CS, Tsai YT, Lo KJ, Lee SD, Chen TS, Lee FY. Medical treatments for bleeding esophageal varices. Hepatology. 1987;7:208. [PubMed] |
| 6. | Reichen J, Le M. Verapamil favorably influences hepatic microvascular exchange and function in rats with cirrhosis of the liver. J Clin Invest. 1986;78:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Reichen J, Hirlinger A, Ha HR, Sägesser S. Chronic verapamil administration lowers portal pressure and improves hepatic function in rats with liver cirrhosis. J Hepatol. 1986;3:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Navasa M, Bosch J, Reichen J, Bru C, Mastai R, Zysset T, Silva G, Chesta J, Rodés J. Effects of verapamil on hepatic and systemic hemodynamics and liver function in patients with cirrhosis and portal hypertension. Hepatology. 1988;8:850-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | García-Pagán JC, Feu F, Luca A, Fernández M, Pizcueta P, Bosch J, Rodés J. Nicardipine increases hepatic blood flow and the hepatic clearance of indocyanine green in patients with cirrhosis. J Hepatol. 1994;20:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Koshy A, Hadengue A, Lee SS, Jiron MI, Lebrec D. Possible deleterious hemodynamic effect of nifedipine on portal hypertension in patients with cirrhosis. Clin Pharmacol Ther. 1987;42:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Casadevall M, Panés J, Piqué JM, Marroni N, Bosch J, Whittle BJ. Involvement of nitric oxide and prostaglandins in gastric mucosal hyperemia of portal-hypertensive anesthetized rats. Hepatology. 1993;18:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Wu Y, Burns RC, Sitzmann JV. Effects of nitric oxide and cyclooxygenase inhibition on splanchnic hemodynamics in portal hypertension. Hepatology. 1993;18:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Theodorakis NG, Wang YN, Skill NJ, Metz MA, Cahill PA, Redmond EM, Sitzmann JV. The role of nitric oxide synthase isoforms in extrahepatic portal hypertension: studies in gene-knockout mice. Gastroenterology. 2003;124:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Lee FY, Colombato LA, Albillos A, Groszmann RJ. Administration of N omega-nitro-L-arginine ameliorates portal-systemic shunting in portal-hypertensive rats. Gastroenterology. 1993;105:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Chan CC, Lee FY, Wang SS, Chang FY, Lin HC, Chu CJ, Tai CC, Lai IN, Lee SD. Effects of vasopressin on portal-systemic collaterals in portal hypertensive rats: role of nitric oxide and prostaglandin. Hepatology. 1999;30:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Mosca P, Lee FY, Kaumann AJ, Groszmann RJ. Pharmacology of portal-systemic collaterals in portal hypertensive rats: role of endothelium. Am J Physiol. 1992;263:G544-G550. [PubMed] |
| 17. | Graupera M, March S, Engel P, Rodés J, Bosch J, García-Pagán JC. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288:G763-G770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Hou MC, Cahill PA, Zhang S, Wang YN, Hendrickson RJ, Redmond EM, Sitzmann JV. Enhanced cyclooxygenase-1 expression within the superior mesenteric artery of portal hypertensive rats: role in the hyperdynamic circulation. Hepatology. 1998;27:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Chan CC, Wang SS, Lee FY, Chang FY, Lin HC, Chu CJ, Chen CT, Huang HC, Lee SD. Endothelin-1 induces vasoconstriction on portal-systemic collaterals of portal hypertensive rats. Hepatology. 2001;33:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Lay CS, Tsai YT, Yang CM, Chen HI, Simchon S, Chien S, Lo KJ. Effect of verapamil on splanchnic haemodynamics in a portal hypertensive rat model. J Gastroenterol Hepatol. 1990;5:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371-G375. [PubMed] |
| 22. | Lay CS, Tsai YT, Kong CW, Lee FY, Chang TT, Lin HC, Yang CM, Lee SD, Chiang BN, Lo KJ. The influence of verapamil and nifedipine on hepatic indocyanine green clearance in patients with HBsAg-positive cirrhosis and ascites. Clin Pharmacol Ther. 1988;44:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Bataller R, Gasull X, Ginès P, Hellemans K, Görbig MN, Nicolás JM, Sancho-Bru P, De Las Heras D, Gual A, Geerts A. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology. 2001;33:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Hamann SR, Blouin RA, McAllister RG Jr. Clinical pharmacokinetics of verapamil. Clin Pharmacokinet. 1984;9:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Sieber CC, Groszmann RJ. Nitric oxide mediates hyporeactivity to vasopressors in mesenteric vessels of portal hypertensive rats. Gastroenterology. 1992;103:235-239. [PubMed] |
| 26. | Pizcueta MP, Piqué JM, Bosch J, Whittle BJ, Moncada S. Effects of inhibiting nitric oxide biosynthesis on the systemic and splanchnic circulation of rats with portal hypertension. Br J Pharmacol. 1992;105:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Niederberger M, Martin PY, Ginès P, Morris K, Tsai P, Xu DL, McMurtry I, Schrier RW. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology. 1995;109:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Pizcueta P, Piqué JM, Fernández M, Bosch J, Rodés J, Whittle BJ, Moncada S. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992;103:1909-1915. [PubMed] |
| 29. | Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |